PUTERI, MITHA DEVINA (2016) UJI NILAI KALOR BAHAN BAKAR SOLAR TERHADAP PERUBAHAN SUHU DAN PENGADUKAN MENGGUNAKAN METODE KALORIMETER BOM (The Experiment Of Diesel Fuel Calorie Toward Change The Temperature And Stirring By Using Calorimeter Bomb Method). Undergraduate thesis, UNDIP.
| PDF (COVER) - Published Version 43Kb | |
| PDF (HAL JUDUl, KATA PENGANTAR, DAFTAR ISI, ABSTRAK) - Published Version 37Kb | |
| PDF (BAB I) - Published Version 9Kb | |
| PDF (BAB II) - Published Version 96Kb | |
| PDF (BAB III) - Published Version 4Kb | |
| PDF (BAB IV) - Published Version 82Kb | |
| PDF (BAB V) - Published Version 13Kb | |
| PDF (BAB VI) - Published Version Restricted to Repository staff only 20Kb | ||
| PDF (BAB VII) - Published Version 7Kb | |
| PDF (DAFTAR PUSTAKA) - Published Version 5Kb | |
| PDF (LAMPIRAN) - Published Version 134Kb |
Abstract
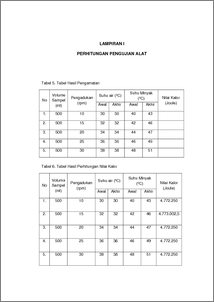
Bomb calorimeter is a device used to measure the amount of heat (calorific value) which was released on combustion (in O2 excess) of a compound, food material, and fuel. Solar is a fuel came fom petroleum processed at the oil refinery and separated the results based on their boiling points so as to produce various of fuels. Usually, solar used for diesel transportation which has a large CC such as truck, tronton, bus, etc. The experiment using by calorimeter bomb with 500 ml solar as a sample to test the calorific value of solar. The principle of bomb calorimeter is carried out under conditions of constant volume without flow or in other the combustion reaction carried out without the use of flame but uses oxygen as the gas burner with a constant volume or high voltage . The result of this test be obtained calorific value from second trialincreased and different from other experiment calorific value. Because when the temperature setting on the second trial only allowed to stand a few minutes from the previous experiments that cause the lack of accurate data is obtained. The calorific value obtained was 4.772.250 J, 4.773.002,5 J, 4.772.250 J, 4.772.250 J,and 4.772.250 J. While the calorific value of diesel theoretically is 4,300,000 J/L . The difference is due to the calorific value of the use of variable temperature is too high so calorific values obtained different calorific value theoretically. For the average values obtained the percent deviation 10.984% and average percent truth obtained was 89.016% . Judging from the truth percent obtained it can be concluded that the bomb calorimeter tools in good condition for the measurement of heat Keyword : Calorimeter bomb, solar, calorific value
| Item Type: | Thesis (Undergraduate) |
|---|---|
| Subjects: | T Technology > TP Chemical technology |
| Divisions: | Faculty of Engineering > Diploma in Chemical Engineering Faculty of Engineering > Diploma in Chemical Engineering |
| ID Code: | 53444 |
| Deposited By: | INVALID USER |
| Deposited On: | 03 May 2017 09:02 |
| Last Modified: | 03 May 2017 09:02 |
Repository Staff Only: item control page