RATNANINGTYAS, FEBRIYANTI (2016) ANALISA PENURUNAN KADAR KLOR DENGAN MENGGUNAKAN ION EXCHANGER PADA AIR POLDER DAERAH TAWANG (Chlorine Levels Decline Analysis by Using Ion Exchanger in Water Regional Polder Tawang). Undergraduate thesis, Undip.
| PDF (COVER, HALAMAN PENGESAHAN, INTISARI, KATA PENGANTAR) - Published Version 235Kb | |
| PDF (DAFTAR ISI) - Published Version 14Kb | |
| PDF (BAB I) - Published Version 157Kb | |
| PDF (BAB II) - Published Version 395Kb | |
| PDF (BAB III) - Published Version 83Kb | |
| PDF (BAB IV) - Published Version 191Kb | |
| PDF (BAB V) - Published Version Restricted to Repository staff only 131Kb | ||
| PDF (BAB VI) - Published Version Restricted to Repository staff only 324Kb | ||
| PDF (BAB VII) - Published Version Restricted to Repository staff only 135Kb | ||
| PDF (DAFTAR PUSTAKA) - Published Version 303Kb | |
| PDF (LAMPIRAN) - Published Version 307Kb |
Abstract
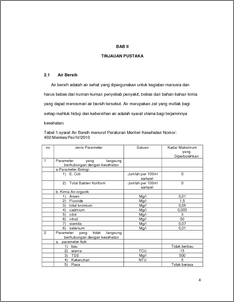
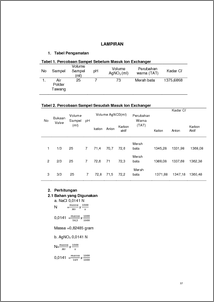
Ion exchange is a physical-chemical process. Ion exchange resin is a polymer having a particular group. Basically the ion exchange resin is divided into two types of cations and anions, where the ability of the ion exchange process is influenced by a large part of the active ingredients contained in the ion exchange resin and capabilities. The concentration of the active site and the ability of a resin ion exchange usually listed in the resin properties. In the process of insoluble compounds, in this case the resin receive a certain positive or negative ions from the solution and releasing other ions into the solution in an equivalent amount of the same. Suite of tools ion exchanger consists of three tanks, the tank cation exchanger, anion exchanger tank, and activated carbon tanks, each of which is made of FRP. In the tank the top there is a way valve to the filter, fast rinse and back wash Chloride ions are formed when the element chlorine gain an electron to form an anion (negatively charged ion) Cl. Chloride ion is one of the major inorganic anions in natural waters are found in greater numbers than other halogen anion. Chloride compounds usually found in the form of sodium chloride (NaCl), potassium chloride (KCl) and calcium chloride (CaCl2). The chloride content analysis using Mohr method.The analyzes were conducted in a neutral atmosphere with a standard solution of silver nitrate and adding a solution of potassium chromate as indicator. The basic principle is the way Mohr brick red precipitate the excess of silver ions with potassium chromate indicator (K2CrO4) which is also the endpoint. Cl levels in the samples out of the tank when the valve opening 1/3 cations; 2/3; and 1 was 1345.28; 1368.08; and 1371.88. In the tank output anion, Cl content to the valve 1/3; 2/3; and 1 was 1331.98; 1337.68; and 1347,18.Kadar Cl at the output of activated carbon tube of variable valve opening 1/3; 2/3; and 1 was 1368.08; 1362.38; and 1360.48. Decreased levels of Cl influenced by the length of time living in the resin sample. Keywords: Ion Exchangers, Mohr method, chloride levels
| Item Type: | Thesis (Undergraduate) |
|---|---|
| Subjects: | T Technology > TP Chemical technology |
| Divisions: | Faculty of Engineering > Diploma in Chemical Engineering Faculty of Engineering > Diploma in Chemical Engineering |
| ID Code: | 48603 |
| Deposited By: | INVALID USER |
| Deposited On: | 15 Apr 2016 16:13 |
| Last Modified: | 15 Apr 2016 16:13 |
Repository Staff Only: item control page