Apriyani, Endang (2004) Pengaruh dealuminasi berganda terhadap kemampuan zeolit wonosari sebagai adsorrben alkil benzen sufonat. Undergraduate thesis, FMIPA UNDIP.
| PDF Restricted to Repository staff only 2064Kb | ||
| PDF 17Kb | |
| PDF 361Kb | |
| PDF 474Kb | |
| PDF 376Kb | |
| PDF 609Kb | |
| PDF 436Kb | |
| PDF Restricted to Repository staff only 503Kb | ||
| PDF 327Kb | |
| PDF 360Kb | |
| PDF 976Kb |
Abstract
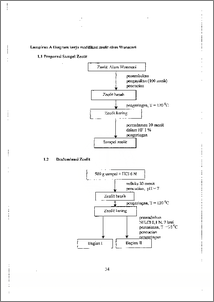
Zeolit alam Wonosari telah dimodifikasi melalui dealuminasi dan kalsinasi supaya dapat digunakan sebagai asorben alkil benzen sulfonat (ABS). Pada tahap pertama, zeolit di.rendam dalam larutan HF 1. % selarna 10 menit kemudian dilanjutkan perendarnan melalui refluks dalam larutan H.C1 6 N selama 30 men.it untuk mernperoleh zeolit I.. Zeolit TI di.buat dengan. perendarnan zeolit melalui refluks dalam HC1 6 N selama. 30 menit d.an dilanjutkan perendarnan. dal.am NH4C1 0,1 N selama tujuh hart. Setelah proses perendaman dilakukan kalsinasi pada suhu 250 °C dan hidrotermal pada suhu 350 uC masing-masing selama 4 jam. Melaluim.etode metil.en blue active substans (MBAS) dapat diketah.ui besatnya surfaktan ABS yang terserap oleh zeolit. Kea` s'aman zeolit diketahui dengan uji keasam.an. Rasio Si/Al. diketahui. dari data AAS. Luas pennukaan dan ukuran pori diperoleh dari data BET. Hasil penelitian menunjukkan untuk zeolit alam, zeolit I, dan. zeolit H masing-masing kea.sarr.tan. 0,628; 0,776; dan 0,909 g/mol. Luas permukaannya masing-masing 10,20; 47,69; dan 14,55 sqm, den.gan rasio Si/Al 4,354; 8,690; dan 8,371. Jari-jari pori rata-rata zeolit sebesar 11,24; 10,58; dan 10,05 A yang menunjukkan terjadinya. penurunan. Data XRD menunjukkan bahwa kristalinitas zeolit m.enurun terlihat dari puncak-puncaknya. Dart MBAS diketahui bahwa kernampuan adsorbsi zeolit menurun, untuk zeolit alam 57,99 %, zeolit 1 50,84 %, dan zeolit II sebesar 46,88 %. Natural zeolite of Wonosari has been modified by dealumination and calcination. The product was used to adsorb alkil benzene sulfonat (ABS). At first, zeolite was immersed in HF 1 % for 1.0 minutes. Delumination was done by refl:ux in HCI. 6 N for 30 minutes to producct zeolite I and continued by immersed in N17140. 0.1 N for seven days to produce zeolite 1I. Zeol.i.te I and II were calcinated in. furnace at 250 °C and hydrothermal treatment at 350 °C for four hours. Metilen blue active substants (MBAS) was used to determine surfactant concentration that has been. adsorbed by zeolite indirectly. Zeolite acidity was determine by acidity test whereas Si/Al ratio was determine by AAS. Surface area and pore volume were determine by -- BET. The result were showed that natural zeolite, zeolite I, and zeolite II had acidity 0.628; 0.776; and 0.909 glinot, surface area were 10.20; 47.69; and 14.55 aim, and Si/Al ratio were 4.354; 8.690; and 8.371. The average pore radius were 11.24; 10.58; and 10.05 A that showed decreasing. XRD data has been known that ze
| Item Type: | Thesis (Undergraduate) |
|---|---|
| Subjects: | Q Science > QD Chemistry |
| Divisions: | Faculty of Science and Mathematics > Department of Chemistry |
| ID Code: | 31016 |
| Deposited By: | Mr UPT Perpus 1 |
| Deposited On: | 11 Nov 2011 13:00 |
| Last Modified: | 11 Nov 2011 13:00 |
Repository Staff Only: item control page