Khanif , Muhammad (2001) Pembuatan mKatalis Ni-Zeonit dengan metode pertukaran Ion. Undergraduate thesis, FMIPA UNDIP.
| PDF Restricted to Repository staff only 1440Kb | ||
| PDF 14Kb | |
| PDF 360Kb | |
| PDF 441Kb | |
| PDF 382Kb | |
| PDF 561Kb | |
| PDF 409Kb | |
| PDF Restricted to Repository staff only 557Kb | ||
| PDF 330Kb | |
| PDF 351Kb | |
| PDF 508Kb |
Abstract
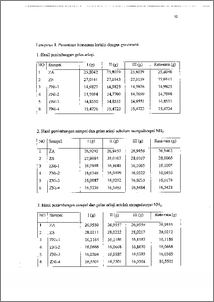
Pembuatan katalis Ni-zeolit telah dilakukan dengan cara pertukaran ion larutan nikel nitrat pada pengemban zeolit alam. Zeolit alam diaktifkan dengan proses dealuminasi, menggunakan HCl 6 N pada ternperatur 90 °C selama 3 jam. Zeolit terdealuminasi diubah menjadi NH4-zeolit kemudian dilakukan proses pertukaran ion dengan Ni 2,5 % berat. Setelah pertukaran ion Ailanjutkan pengeringan, kalsinasi dan aktivasi. Variabel yang ditinjau adalah pengaruh variasi konsentrasi ion amonium terhadap jumlah logam Ni dalam zeolit. Karakterisasi katalis dilakukan dengan AAS yang bertujuan untuk menentukan kadar logam. Keasaman katalis ditentukan melalui metode adsorpsi gas NH3 yang ditentukan secara gravimetri dan spektroskopi FTIR. Juga dilakukan kajian terhadap hubungan antar parameter yaitu: konsentrasi ion amonium, kadar logam, dan nilai keasaman. Hasil penelitian menunjukkan bahwa konsentrasi ion amonium 2,0 M mendapatkan katalis terbaik dengan kadar logam Ni 3186,898 ppm dan keasaman sebesar 1,0697 mmol g-1. Hasil analisa spektroskopi FTIR menunjukkan zeolit alam mengadsorpsi NH3 pada bilangan gelombang 1.385,8 cm -1 dan katalis Ni zeolit mengadsorpsi NH3 pada bilangan gelombang 1.400,2 cm-1. Jumlah logam Ni yang terdispersi dalam rongga zeolit dipengaruhi oleh konsentrasi ion amonium sedangkan nilai keasaman katalis dipengaruhi oleh jumlah logam Ni Preparation of Ni-zeolite catalyst by ion exchange nickel nitrate solution into natural zeolite support has been conducted. Natural zeolite was activated by process of dealumination, using HC1 6 N at 90 °C for 3 hours. Dealuminated zeolite was exchange into NH4-zeolite then ion exchange with 2.5 wt % Ni. After ion exchange process it was followed by drying, calcination and activation procedures. Variable observed was the effect of various concentration of ammonium ion on Ni content in zeolite. The characterization of catalyst was performed by AAS in order to determine nickel content. Catalyst acidity was evaluated by ammonia gas adsorption according to techniques of gravimetry and spectroscopy FTIR. It was also studied the relation of some parameters e.g. the ammonium ion concentration, metal content, and catalyst acidity. The result showed that concentration of ammonium ion of 2.0 M produced the best catalyst which contained Ni metals of 3186.898 ppm and acidity number of 1.0679 mmol FTIR spectra showed that natural zeolite adsorbed NH3 at 1385.8 cm-I and Ni-Zeolite catalyst adsorbed NH3 at 1400.2 cm-1 The amount of Ni metals which dispersed into zeolite cavity was affected by the ammonium ion concentration meanwhile catalyst acidity was affected by Ni content.
| Item Type: | Thesis (Undergraduate) |
|---|---|
| Subjects: | Q Science > QD Chemistry |
| Divisions: | Faculty of Science and Mathematics > Department of Chemistry |
| ID Code: | 30800 |
| Deposited By: | Mr UPT Perpus 1 |
| Deposited On: | 07 Nov 2011 11:15 |
| Last Modified: | 07 Nov 2011 11:15 |
Repository Staff Only: item control page