Wijayanto , Wahyu (1999) Sintesis dan karakterisasi Katalis Ni-Zeolit. Undergraduate thesis, FMIPA Undip.
| PDF Restricted to Repository staff only 1707Kb | ||
| PDF 14Kb | |
| PDF 346Kb | |
| PDF 440Kb | |
| PDF 371Kb | |
| PDF 695Kb | |
| PDF 381Kb | |
| PDF Restricted to Repository staff only 595Kb | ||
| PDF 326Kb | |
| PDF 350Kb | |
| PDF 566Kb |
Abstract
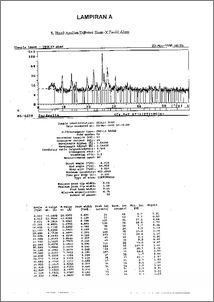
Pemanfaatan zeolit alam sebagai pengemban dalam pembuatan katalis hidrokraking telah dilalcukan. Aktivasi zeolit alam dengan perlakuan HC1 6 N pada temperatur 100°C selama 3 jam. Pemasukkan logam pada pengemban dilakukan dengan metode ion exchange larutan Ni(NO3)2.6H20 ( 2% berat ), kemudian dikalsinasi dan direduksi. Karakterisasi katalis dilakukan dengan mengunakan difraksi sinar-x untuk menentukan kristalinitas, sifat keasaman dengan adsorpsi uap amonia secara gravimetri dan spektrofotometri infra merah. Analisa difraksi sinar-x menunjukkan sampel mengandung tipe kristal mordenit. Proses perlakuan asam, pemasukkan logam nikel dan perlakuan termal dapat meningkatkan keasaman total zeolit alam ( ZA ) dari 2,49 mmol.g-1 menjadi 2,862 mmol.g-I ( Ni-ZR-450 ). Proses alctivasi mampu meningkatkan kristalinitas katalis dan mencapai maksimum pada sudut 24 = '25,.5`78 . Utilize natural zeolites as support in hidrocracking catalyst preparation has been done. Natural zeolites were treated with 6 N HCl at 100°C for 3 hours. The loading of metal into support was carried out fraught Ni(NO3)2.6H20 ( 2 w.t. % ) by ion exchange. Its followed with drying calcination and reduction. Characterization of catalyst was conducted by X-ray diffraction to determine crystallinity, acidity with adsorption of amonia vapour according to gravimetry and infra red spectrophotometry. X-ray diffraction analysis samples indicated that mainly consist of mordenit type crystallin matter. Acid treatment, the loading nickel metal and thermal treatment processes can improve the acidity of natural zeolites ( ZA ) from 2,49 nunol.g-1 to 2,862 mmol.g-1 ( Ni-ZR-450 ). Activation process can improve crystallinity of catalyst and reached at 213 angle = 25,598 °.,
| Item Type: | Thesis (Undergraduate) |
|---|---|
| Subjects: | Q Science > QD Chemistry |
| Divisions: | Faculty of Science and Mathematics > Department of Chemistry |
| ID Code: | 30738 |
| Deposited By: | Mr UPT Perpus 1 |
| Deposited On: | 05 Nov 2011 11:17 |
| Last Modified: | 05 Nov 2011 11:17 |
Repository Staff Only: item control page