KHIQMAH, NUR VENTIRA (2015) Pengembangan Teknik Adsorpsi dengan Menggunakan Ion Exchanger Berbasis Zeolit-Karbon Aktif untuk Produksi Air Sanitasi (Development of Adsorption Techniques Using the Ion Exchanger Zeolite-Based Activated Carbon for the Production Sanitation Water). Undergraduate thesis, Undip.
| PDF (COVER, HALAMAN PENGESAHAN) - Published Version 186Kb | |
| PDF (BAB I) - Published Version 85Kb | |
| PDF (BAB II) - Published Version 195Kb | |
| PDF (BAB III) - Published Version 83Kb | |
| PDF (BAB IV) - Published Version 243Kb | |
| PDF (BAB V) - Published Version Restricted to Repository staff only 28Kb | ||
| PDF (BAB VI) - Published Version Restricted to Repository staff only 305Kb | ||
| PDF (BAB VII) - Published Version Restricted to Repository staff only 84Kb | ||
| PDF (DAFTAR PUSTAKA) - Published Version 147Kb | |
| PDF (LAMPIRAN) - Published Version 544Kb | |
| PDF (KATA PENGANTAR, DAFTAR ISI) - Published Version 163Kb |
Abstract
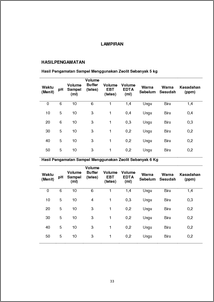
Hard water is water that contains salts of calcium and magnesium in large enough quantities. These salts are usually in the form of bicarbonate salts, chlorides, sulfates and nitrates. Calcium and magnesium compounds react with soap to form sludge and prevent foam in water. Therefore, the compounds of calcium and magnesium is relatively poorly soluble in water, the compounds tend to separate from the solution in the form of sludge or precipitates which eventually became crust. In use long enough hard water can cause kidney disorders due to sediment accumulation of CaCO3 and MgCO3 in the kidney. To obtain fresh water suitable for consumption in need of a better way of processing. One widely used method is filtration (filter) This method can be applied in rural areas that are on the edge of a river or other water source. Filtration media normally used are sand, gravel, fibers, activated charcoal and zeolite. In Indonesia zeolite apparently has not received adequate attention as a clean water filtration media. Though Indonesia is geographically located on the path that has the potential volcanic zeolite is large enough. In order to study alternative methods to reduce water hardness, in this study used natural zeolite as an ion exchanger (ion exchange). The principle of ion exchanger is in the process of insoluble compounds, in this case the resin, receive a certain positive or negative ions from solution and release other ions into the solution in an amount equivalent to the same. If the ions are exchanged in the form of a cation, then the resin is called cation exchange resin, and if ions are exchanged in the form of anion, the resin is called an anion exchange resin. The series of ion exchangers tool consists of three tanks, the tank cation exchanger, anion exchanger tank, and activated carbon tanks, each of which is made of FRP. At the top of the tank there is a way valve to the filter, fast rinse and back wash. The method used in the analysis of the levels of Ca and Mg is complexometry titration is the method in which the titrant and titratnya each other to form neutral complexes that dissociate in solution. In this lab to test the hardness is done by means of Ion Exchanger and Ion Exchangers combination of activated carbon, with a total of 110 liters of water 10 ml samples were taken every 10 minutes for 6 times. Results of observations obtained for impairment hardness USING combination of zeolite 6 kg and activated carbon which is 1.4 ppm, 03 ppm, 0.2 ppm, 0.2 ppm, 0.2 ppm, and 0.2 PMM. As for the results of the impairment hardness with a combination of zeolite ion exchanger 5 kg of activated carbon obtained 1.4 ppm, 0.4 ppm, 0.3 ppm, 0.2 ppm, 0.2 ppm, 0.2 ppm. It can be concluded using a combination of zeolite Ion Exchanger 6 kg and activated carbon is more effective, because the function of activated carbon as an adsorbent that is able to absorb Ca and Mg ions contained in the water. (Hardness, ion exchangers, zeolites, resin)
| Item Type: | Thesis (Undergraduate) |
|---|---|
| Subjects: | T Technology > TP Chemical technology |
| Divisions: | Faculty of Engineering > Diploma in Chemical Engineering Faculty of Engineering > Diploma in Chemical Engineering |
| ID Code: | 48040 |
| Deposited By: | INVALID USER |
| Deposited On: | 18 Mar 2016 08:31 |
| Last Modified: | 18 Mar 2016 08:31 |
Repository Staff Only: item control page