Khasanan , Rokhmatun (2005) Desalinasi air laut menggunakan membran cair berpendukung (SLM). Undergraduate thesis, FMIPA UNDIP.
| PDF Restricted to Repository staff only 2019Kb | ||
| PDF 23Kb | |
| PDF 373Kb | |
| PDF 464Kb | |
| PDF 395Kb | |
| PDF 553Kb | |
| PDF 423Kb | |
| PDF Restricted to Repository staff only 641Kb | ||
| PDF 320Kb | |
| PDF 377Kb | |
| PDF 556Kb |
Abstract
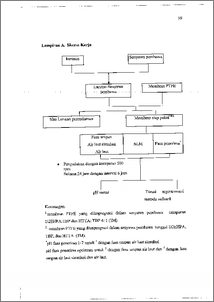
Salah satu cara untuk memperoleh air bersih adalah dengan menghilangkan garam dari air laut atau desalinasi. Teknik desalinasi yang biasa dilakukan antara lain dengan osmosis balik, penukar ion, dm. elektrodialisis. Membran cair berpendukung (SLM) menawarkan suatu alternatif barn untuk proses desalinasi. Teknik SLM merupakan teknik pemisahan dimana fasa organik yang tidak saling bercampur dengan fasa air. Fasa organik dan senyawa pembawa diimpregnasikan ke dalam membran berpori dan diletakkan diantara dua fasa air yaitu fasa umpan dan fasa penerima. Penelitian ini bertujuan untuk mengamati penurunan kadar salinitas sampel air laut menggunakan teknik SLM. Desalinasi air laut dengan teknik SLM dipelajari menggunakan membran berpendukung polytetrafluoroetilen (PTFE) yang mengandung di-2-ethylhexyl phosphoric acid (D2EHPA), thenoyltrifluoroacetone (HTTA), tributhyl phosphate (TBP), dan campuran D2EHPA-TBP, HTTA-TBP dengan perbandingan konsentrasi masing-masing 4: 1. pH fasa penerima divariasi pada pH 1-7. Waktu pengadukan 24 jam dengan interval waktu pengambilan sampel tiap 6 jam. Penurunan kadar salinitas ditentukan dengan titrasi argentometri metode Volhard. Hasil penelitian diperoleh penurunan kadar salinitas (persen desalinasi) air laut terbesar pada pH fasa penerima ± 5. Persen desalinasi air laut pada pH tersebut berturut-turut untuk senyawa pembawa tunggal TBP; D2EHPA; HTTA adalah 10,39 %; 31,169 %; 54,026 %. Untuk senyawa pembawa campuran D2EHPA-TBP; HTTA-TBP, dengan perbandingan 4 : 1 adalah 81,69 %; 84,507%. Berdasarkan hasil penelitian tersebut, dapat diketahui bahwa senyawa pembawa campuran memberikan efek sinergi pada proses desalinasi dengan teknik SLM yang ditunjukkan dengan peningkatan persen desalinasi yang cukup besar. Desalination is one of the methods to get fresh water by reducing salt content from seawater. Reverse osmosis, ion exchange, and electro dialysis are included in desalination techniques. Supported liquid membrane (SLM) gives a new alternative for desalination process. It is a separation technique were organic and water phases are immiscible each other. Organic phases has a carrier compound are impregnated into pore membrane and the membrane is put between two phases of water that are feed and stripping phases. This research observed decreasing salinity of seawater sample using SLM technique. Desalination of seawater used SLM technique by supported membrane of polytetrafluoroetilen (PTFE) that containing di-2-ethylhexyl phosphoric acid (D2EHPA), thenoyltrifluoroacetone (HTTA), tributhyl phosphate (TBP), and mixture of D2EHPA-TBP, HTTA-TBP (with concentration ratio 4 : 1) respectively was studied. pH of stripping phase was adjusted at pH 1-7. Stirrer time was 24 hours with interval every 6 hours. Reducing salinity content was determined by argentometric Volhard method titration. Result of this research showed that reducing salinity content (desalination percent) was maximums at pH of stripping phase ± 5. And the desalination percent of seawater at pH off 5 were 10, 39 %; 31,169 %; 54,026 %, for carrier compounds of TBP; D2EHPA; HTTA respectively. For carrier compound mixtures of D2EHPA-TBP, HTTA-TBP, with concentration ratio 4 : 1 were 81,69 %; 84,507 %. Based on that result, it could be concluded that carrier compound mixtures gave a synergy effect to desalination process with SLM, as shown by increasing desalination percent.
| Item Type: | Thesis (Undergraduate) |
|---|---|
| Subjects: | Q Science > QD Chemistry |
| Divisions: | Faculty of Science and Mathematics > Department of Chemistry |
| ID Code: | 31086 |
| Deposited By: | Mr UPT Perpus 1 |
| Deposited On: | 14 Nov 2011 14:23 |
| Last Modified: | 14 Nov 2011 14:23 |
Repository Staff Only: item control page