Pratiwi , Ika Budi (2005) Peningkatan kadar kalium (K) pada Feldspar dengan metode pertukaran Ion sistem kolom. Undergraduate thesis, FMIPA UNDIP.
| PDF Restricted to Repository staff only 1820Kb | ||
| PDF 30Kb | |
| PDF 349Kb | |
| PDF 440Kb | |
| PDF 371Kb | |
| PDF 476Kb | |
| PDF 372Kb | |
| PDF Restricted to Repository staff only 591Kb | ||
| PDF 319Kb | |
| PDF 347Kb | |
| PDF 448Kb |
Abstract
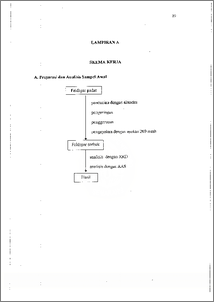
Feldspar banyak digunakan sebagai bahan pelebur pada industri keramik, salah satu syarat utarnanya adalah kadar kalium di dalamnya harus Iebih tinggi daripada kadar kalsium. Mineral feldspar yang banyak terdapat di Indonesia menunjukkan hal yang sebaliknya, sehingga perlu diolah agar kadar kaliumnya meningkat. Dalam penelitian ini dilakukan peningkatan kadar kalium pada feldspar dengan metode pertukaran ion sistem kolom. Pertukaran ion sistem kolom dilakukan dengan memvariasi pH dan konsentrasi KC1 yang digunakan. Jenis mineral yang terkandung dalam feldspar dikarakterisasi menggunakan metode X-ray difi-action (XRD), sedangkan banyaknya kation K+ dan Ca2+ yang tertukar ditentukan dengan metode tak langsung yaitu dengan menganalisis filtrat. hasil pertukaran dengan spelctrofotometri serapan atom. Kondisi optimum pertukaran ion dicapai pada pH 11 dan konsentrasi KC1 15000 ppm dengan peningkatan kadar kalium sebesar 1,070 mek dan pengurangan kadar kalsium sebesar 1,15.10 mek. Feldspar is well known as a melting material at ceramics industries, the main specification is that the content of potassium must be higher than the calsium. On the other hand, Indonesian feldspar contains much calsium and lack of potassium so that ion exchange column system is used to increase the potassium content of the feldspar. The ion exchange column system was done by preparing the various pH and concentration of KCI. The type of minerals contained in feldspar had been characterized by XRD, whereas the changes of K+ and Cat concentrations were measured by analyzing the filtrate on undirect method by Atomic Absorption Spectrophotometiy (AAS). Optimum condition of the ion exchange was reached at the concentration of KCI and pH, 15000 ppm and 11 respectively. In that condition the increase of potassium content was 1.070 mek while the decrease of calcium content was 1.15.104 mek. vi This dlocument is Undip Institutional Repository Collection. The author(s) or copyright owner(s) agree that UNDIP-IR may, without changing the content, translate the1 submission to any medium or format for the purpose of preservation. The author(s) or copyright owner(s) also agree that UNDIP-IR may keep more than one copy of thisl 1 submIssion for purposes of security, back-up and preservation. ( http://eprints.undip.ac.id)
| Item Type: | Thesis (Undergraduate) |
|---|---|
| Subjects: | Q Science > QD Chemistry |
| Divisions: | Faculty of Science and Mathematics > Department of Chemistry |
| ID Code: | 31072 |
| Deposited By: | Mr UPT Perpus 1 |
| Deposited On: | 14 Nov 2011 10:54 |
| Last Modified: | 14 Nov 2011 10:54 |
Repository Staff Only: item control page