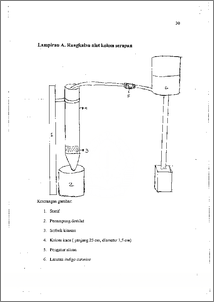
Setyadi , Edi (2005) Adsorbsi zat warna indigi cermine oleh kitosen dengan metode kolom. Undergraduate thesis, FMIPA UNDIP.
| PDF Restricted to Repository staff only 1543Kb | ||
| PDF 15Kb | |
| PDF 360Kb | |
| PDF 452Kb | |
| PDF 390Kb | |
| PDF 669Kb | |
| PDF 406Kb | |
| PDF Restricted to Repository staff only 562Kb | ||
| PDF 328Kb | |
| PDF 357Kb | |
| PDF 481Kb |
Abstract
Kitosan merupakan suatu senyawa makromolekul berantai panjang yang tersusun dart monomer glukosamin dan dapat diekstrak dart kulit udang dengan proses yang sederhana, Kitosan dapat dimanfaatkan sebagai adsorben zat wama karena memiliki gugus aktif berupa gugus amina dan hidroksi yang dapat berinteraksi dengan zat warna. Tujuan penelitian ini adalah untuk mengetahui pH terbaik, kemampuan adsorpsi dan interaksi antara kitosan dengan indigo carmine pada metode kolom Tetah dilakukan ekstraksi kitosan dart kulit udang dan pernanfaatanya untuk adsorpsi zat warna indigo carmine dengan metode kolom. Penalitian dilakukan dengan pemvariasian pH dan konsentrasi larutan indigo carmine. Hash' ekstraksi diperoleh serbuk kitosan berwarna putih kecoklatan dengan derajlt deasetilasi sebesar 62,537%. Hasil adsorpsi menunjukkan bahwa adsorpsi lebih baik pada kondisi asam dan kenaikan pH akan menurunkan kemampuan adsorpsi. Kemampuan adsorpsi terbesar terjadi pada konsentrasi indigo carmine 40 ppm dengan indigo carmine teradsorpsi sebanyak 0,519 mg/g. Interaksi yang terjadi antara kitosan dan indigo carmine disebabkan oleh adanya gaya elektrostatik yang timbul karena perbedaan muatan antara kitosan dan indigo carmine. Chitosan is a macromolecules compound whith the glucosamine monomer in some extent. Chitosan can be extracted from shrimp with simple process. Chitosan can be used as dyes adsorbent because of its active groups of amine and hydroxi that would interact with dyes. The aim of research are to know the best pH on adsorption, ability and to evaluate the interaction between chitosan and indigo carmine. Extraction chitosan from shrimp an also use for adsorption indigo carmine dye by column method have been conducted. Research done with variation of pH and concentration of indigo carmine dye at column method. Experiment result in brownich white Chitosan powder with degree of deacetilation is 62,537%. Evaluation of its adsorption ability show that the better adsorption at acid condition and increasing of pH will decrease ability of adsrption. Biggest adsorption ability happened at concentration 40 ppm wish indigo carmine adsorbed is 0,519 mg/g. Interaction between chitosan and indigo carmine was caused by electrostatic force which arising from its difference charge between chitosan and indigo carmine. vi
| Item Type: | Thesis (Undergraduate) |
|---|---|
| Subjects: | Q Science > QD Chemistry |
| Divisions: | Faculty of Science and Mathematics > Department of Chemistry |
| ID Code: | 31048 |
| Deposited By: | Mr UPT Perpus 1 |
| Deposited On: | 14 Nov 2011 08:35 |
| Last Modified: | 14 Nov 2011 08:35 |
Repository Staff Only: item control page