Susanto , Heri (2004) Sintesis garam N-isoprofil yohimbinium iodida. Undergraduate thesis, FMIPA UNDIP.
| PDF Restricted to Repository staff only 1166Kb | ||
| PDF 15Kb | |
| PDF 361Kb | |
| PDF 462Kb | |
| PDF 376Kb | |
| PDF 402Kb | |
| PDF 346Kb | |
| PDF Restricted to Repository staff only 546Kb | ||
| PDF 326Kb | |
| PDF 331Kb | |
| PDF 378Kb |
Abstract
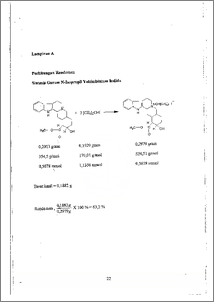
Metode transformasi amina tersier masih dikembangkan di Laboratorium Kimia Organik Universitas Diponegoro melalui alkilasi terhadap metil-amina¬tersier membentuk garam metil-alkil-amina-tersier (tahap I), diikuti demetilasi spesifik membentuk alkil amina tersier (tahapII). Sebagai aplikasi awal dari metode tersebut, pada penelitian ini telah dilakukan kuarternerisasi terhadap senyawa alkaloid alami, yakni yohimbin. Dilihat clan strukturnya, senyawa yohimbin memiliki dua pusat aktif yang berpotensi untuk diserang reagen pengalkil, yakni sistem bisiklik lingkar enam dan sistem pirola. Dengan perbandingan mol 1:2, antara yohimbin dan isopropil iodida dalam refluks metanol selama 18 jam, telah menghasilkan produk transformasi garam yohimbinium sebanyak 63,2 % dengan titik leleh 269 °C Keberadaan senyawa target telah dapat dibuktikan dengan spektroskopi 11-1-NMR. Dengan menghitung dan membandingkan luas puncak pada daerah¬daerah pergeseran kimia tertentu, telah dapat dibuktikan bahwa isopropil iodida menyerang pada salah sate sistem amina yakni atom N pada sistem bisiklik Iebih reaktif daripada atom N .pada sistem pirola. Dari perhitungan dengan perbandingan luas puncak diketahui bahwa senyawa target adalah N-isopropil yohimbinium iodida. Tertiery Amine transformation method has been developed in Organic Chemistry Laboratory of Diponegoro University by alkilation of methyl-tertiary¬amine forming methyl-alkyl-quarternary ammonium (first step), followed by spesific demetilation to produce alkyl-tertiary amine (second step). As a first application of this method, this research has been done quarternerization of yohimbine, one of natural alcaloide compound. From it's structur, yohimbine compound have two active sides that potential to be attacted by alkylation reagent. The active sides is six cycle of bicyclic system and pyrola system. With mole comparation 1:2 between yohimbine and isopropyl iodide in methanol reflux for 18 hours, was produce yohimbinium salt, the transformation product for 63,2 % in yield and the melting point 269 °C. The target compound existance could be confirmed by 1H-NMR spectroscopy. With estimating and comparasing of wide peaks in spesific chemical shiff, shown that isopropyl iodide was bonded in one ammines system only, that was in atom N-bicyclic that more reactive than atom N in pyrola's system.The estimation of wide peaks resulted that the target compound is N-isopropyl yohimbinium iodide
| Item Type: | Thesis (Undergraduate) |
|---|---|
| Subjects: | Q Science > QD Chemistry |
| Divisions: | Faculty of Science and Mathematics > Department of Chemistry |
| ID Code: | 31019 |
| Deposited By: | Mr UPT Perpus 1 |
| Deposited On: | 11 Nov 2011 13:12 |
| Last Modified: | 11 Nov 2011 13:12 |
Repository Staff Only: item control page