Cahyadi , Nur (2004) Pengaruh variasi komposisi dan waktu sintering terhadap konduktivitas elektrolit padat sistem Bi203-Cao. Undergraduate thesis, FMIPA UNDIP.
| PDF Restricted to Repository staff only 1835Kb | ||
| PDF 17Kb | |
| PDF 354Kb | |
| PDF 495Kb | |
| PDF 414Kb | |
| PDF 608Kb | |
| PDF 379Kb | |
| PDF Restricted to Repository staff only 575Kb | ||
| PDF 328Kb | |
| PDF 347Kb | |
| PDF 695Kb |
Abstract
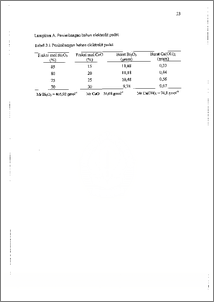
Telah dibuat elektrolit padat sel bahan bakar Bi203-Ca0 dengan variasi persen mol Ca0 pada 15, 20, 25, dan 30 %. Pembuatan elektrolit dilakukan dengan mencampur Bi203 dan Ca(OH)2, kemudian campuran dikalsinasi pada suhu 700 °C selama I jam, campuran dicetak selanjutnya disinter pada 850 °C dengan variasi waktu 7 dan 10 jam. Jenis fasa yang'terbentuk dalam elektrolit padat yang dihasiikan ditentukan dengan difraktometer sinar-X, densitas, dan porositas ditentukan dengan metode Archimedes, sedang konduktivitas ditentukan dengan metode DC. Jenis fasa yang terbentuk adalah fasa 6-Bi203, 3-Bi203, dan fasa stoikiometri Bi14Ca5026, fasa-fasa tersebut terbentuk pada sampel dengan persen mol Ca0 20, 25, dan 30 %. Peningkatan rasio komposisi Bi203-Ca0 tidak membe`rikan pola kecenderungan yang tetap terhadap konduktivitas sampel, seciangkah peningkatan waktu sintering meningkatkan konduktivitas sampel. Sampel terbaik adalah sampel dengan persen mol Ca0 25 % dengan waktu sintering 7 jam, konduktivitas sampel tersebut pada suhu 400 °C adalah 1,04x10-5 S cm-I. Bi203-CaO Fuel Cell solid electrolyte has been prepared with varied CaO concentration at 15, 20, 25, and 30 mole % to study the influence of composition variation and sintering time on the conductivity of the electrolyte. Electrolyte was made by mixing Bi203 and Ca(OH)2 in water medium then calculated at 700 °C for 1 hour. The mixture then molded and sintered at 850 °C for 7 and 10 hours. The products were characterized using X-ray diffractometer to determine the phases formed. The Archimedes method was used for density and porosity measurement while the DC method used for conductivity measurement. There have been 8-Bi203, p-Bi203, and stoichiometric phase Bi14Ca5026 formed in electrolytes with CaO concentration at 20, 25, and 30 mole %. Decrease of Bi203 ratio to CaO has not given any obvious tendency pattern to conductivity. Increase of sintering time from 7 to 10 hours has increased conductivity of the electrolyte. The best sample was electrolyte with CaO concentration 25 mole %, sintered for 7 hours, and the conductivity equal to 1,04x10-5 S cm-1 at 400°C.
| Item Type: | Thesis (Undergraduate) |
|---|---|
| Subjects: | Q Science > QD Chemistry |
| Divisions: | Faculty of Science and Mathematics > Department of Chemistry |
| ID Code: | 30997 |
| Deposited By: | Mr UPT Perpus 1 |
| Deposited On: | 11 Nov 2011 08:19 |
| Last Modified: | 11 Nov 2011 08:19 |
Repository Staff Only: item control page