P a t m a , P a t m a (2004) Transformasi senyawa N-Metilpiperidin. Undergraduate thesis, FMIPA UNDIP.
| PDF Restricted to Repository staff only 1281Kb | ||
| PDF 15Kb | |
| PDF 377Kb | |
| PDF 418Kb | |
| PDF 394Kb | |
| PDF 508Kb | |
| PDF 352Kb | |
| PDF Restricted to Repository staff only 523Kb | ||
| PDF 324Kb | |
| PDF 349Kb | |
| PDF 333Kb |
Abstract
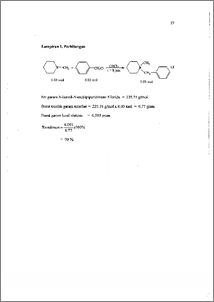
Amina tersier merupakan salah satu golongan senyawa amina yang penting dalam kehidupan manusia, terutama di bidang pertanian dan kedokteran. Penggantian salah satu atau beberapa gugus yang terikat dapat menimbulkan aktifitas yang berbeda. Penelitian tentang hal ini telah banyak dilakukan untuk mengetahui hubungan antara struktur dengan aktifitas yang dimiliki oleh senyawa. Transformasi amina tersier untuk memperoleh senyawa turunannya yang Iebih potensial dapat dilakukan melalui dua tahap, yaitu pembentukan garam ammonium kuaterner melalui reaksi alkilasi dan dekuaternerisasi garam ammonium kuatemer dengan nukleofil eksternal. Trifenilfosfin sebagai nukleofil eksternal yang bersifat netral yang efektif digunakan dalam reaksi dekuaternerisasi garam amonium aromatik, diharapkan memiliki efektifitas yang sama untuk garam amonium non aromatik. Melalui penelitian ini diharapkan dapat diketahui peranan trifenilfosfin dan pengaruh gugus benzil dalam reaksi dekuaternerisasi garam N-benzil-N¬metilpiperidinium klorida. Pada tahap awal, telah berhasil disintesis garam amonium, yaitu garam N-benzil-N-metilpiperidinim klorida melalui reaksi benzil klorida dengan metil piperidin dalam retiuks kloroform selama 8 jam. Garam ini diperoleh secara kuantitatif sebesar 90 % dan dapat dibuktikan melalui data 11-1-NMR. Pada tahap kedua dilakukan dekuaternerisasi garam N-benzil-N-metilpiperidinim klorida menggimakan pelarut DMF, selama 18 jam pada temperatur 153 °C. Dari hasil analisa 1H-NMR dan GC-MS diperoleh adanya trifenilfosfin awal dan garam amonium tidak ditemukan. Melalui kenyataan ini dapat diusulkan bahwa telah terjadi autodekuaternerisasi dengan nukleofil internal, yaitu ion klorida. Tertiary amine represent one of the important amine compounds faction in human life especially in the field of agriculture and medical. Replacement one or some bunch can generate different activity. Researchs on the effect have been conducted knowing relation among structure and activity from its compound. Transformation of tertiary amine was done by two phase, that is forming quartenery ammonium salt through reaction of alkylation and dequarteneritation of quartenery ammonium salt with external nucleophyle. Triphenylpho spine as external nucleophyle have neutral character which is effective to be used in reaction of dequarteneritation from the aromatic ammonium salt. And it is expected to have same effectivity for the non aromatic ammonium salt. This research is conducted knowing the role of triphenylphospine and the influence of benzyl bunch in reaction of dequarteneritation from N-benzil-N¬mefflpiperidinium chloride salt. Transformation of amines particularly methyl piperidine, through quaternerisation process with benzyl chloride was examined, followed by dequaternerisation using triphenylphosphine (PPh3) as nucleophyle. In the first step, N-benzyl-N-metilpiperidinium chloride salt was synthesis by reaction of benzyl chloride with methyl piperidinium in reflux of CHC13 for 8 hours. This ammonium salt has been obtained quantitatively 90 % of yield by At conducted both of N-Benzil-N-metilpiperidinium chloride salt dequaternerisation use MAY, during 18 hour at temperature 153 °C. From result of analysis 1H-NIVIR and GC-MS obtained by the existence of triphenylphosphine early and amonium salt do not be found. Through this fact can be suggested that by have happened reaction of autodequaternerisation with internal nucleophyle, that is chloride ion.
| Item Type: | Thesis (Undergraduate) |
|---|---|
| Subjects: | Q Science > QD Chemistry |
| Divisions: | Faculty of Science and Mathematics > Department of Chemistry |
| ID Code: | 30996 |
| Deposited By: | Mr UPT Perpus 1 |
| Deposited On: | 11 Nov 2011 08:15 |
| Last Modified: | 11 Nov 2011 08:15 |
Repository Staff Only: item control page