Syafana , Yulia (2004) Elektrolisis larutan perak diamina dengan variasi kuat arus. Undergraduate thesis, FMIPA UNDIP.
| PDF Restricted to Repository staff only 1365Kb | ||
| PDF 15Kb | |
| PDF 348Kb | |
| PDF 439Kb | |
| PDF 383Kb | |
| PDF 568Kb | |
| PDF 409Kb | |
| PDF Restricted to Repository staff only 538Kb | ||
| PDF 323Kb | |
| PDF 335Kb | |
| PDF 383Kb |
Abstract
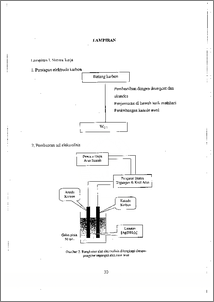
Penelitian ini bertujuan untuk menguji ketaatan elektrolisis larutan perak diamina, [Ag(NH3)2](aq), dengan memvariasi kuat arus terhadap hukum Faraday W = e i t, selain memperoleh endapan perak yang mengkilap dengan efisiensi mendekati 100 %. Komposisi elektrolit mempengaruhi produk elektrolisis perak yang dihasilkan. Telah dilakukan elektrolisis pengendapan perak dari larutan perak diamina dengan variasi kuat arus dari 1, 2, 3, hingga 10 miliampere. Sel elektrolisis dijalankan pada temperatur kamar selama dua jam dengan potensial listrik konstan sebesar 1300 millivolt. Perubahan berat katoda sebelum dan sesudah elektrolisis digunakan untuk menghitung berat endapan perak (Wobs) dan efisiensi elektrolisis (Bc). Hasil penelitian menunjukkan bahwa sistem elektrolisis mentaati hukum Faraday, endapan yang dihasilkan berwarna putih mengkilap seperti yang diharapkan dengan efisiensi tneneapai 99,38 % pada kuat arus 2 miliampere. The obedient of AgCl-NH40H(aq) system to Faradays Law under the electric current variation has been examined. In addition, to get shiny silver deposit and efficiency approached to 100 %. Electrolyte composition influenced the electrolysis product. Electrolysis was carried out to precipitate silver from [Ag(NH3)2]+ solution with varied current. Electrolytic cell operated at ambient temperatur for 2 hours with constant applied potential at 1300 mV, while the current varied from 1, 2, 3, to 10 mA. Change of cathode's weight before and after electrolysis used to measure weight of silver and electrolysis efficiency. The result showed the obedience of the system to the Faraday's. law. Shiny silver deposit was obtained as it expected and efficiency of 99.38 % was reached at current 2 MA. 771-1; document is Undip Institutional Repository Collection. The author(s) or copyright owner(s) agree th., ,,,L,I, -h, s—,, ...„ — —,.. t,.. „, ...,- —.,...nt, translate tie submission to any medium or format for the purpose of preservation. The author(s) or copyright owner(s) also agree that UNDIP-IR may keep more than one copy of t1is submission for purposes of security, back-up and preservation. ( http://eprints.undip.ac.id) I I
| Item Type: | Thesis (Undergraduate) |
|---|---|
| Subjects: | Q Science > QD Chemistry |
| Divisions: | Faculty of Science and Mathematics > Department of Chemistry |
| ID Code: | 30980 |
| Deposited By: | Mr UPT Perpus 1 |
| Deposited On: | 10 Nov 2011 15:07 |
| Last Modified: | 10 Nov 2011 15:07 |
Repository Staff Only: item control page