Imron , Ahmad (2003) Modifikasi pori zeolit alam menggunakan lauril benzil dimetil amonium klorida sebagai molekul pengarah. Undergraduate thesis, FMIPA UNDIP.
| PDF Restricted to Repository staff only 1535Kb | ||
| PDF 16Kb | |
| PDF 351Kb | |
| PDF 458Kb | |
| PDF 383Kb | |
| PDF 573Kb | |
| PDF 374Kb | |
| PDF Restricted to Repository staff only 469Kb | ||
| PDF 324Kb | |
| PDF 355Kb | |
| PDF 611Kb |
Abstract
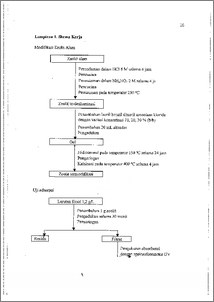
Telah dilakukan modifikasi terhadap pori zeolit alam dengan lauril benzil dimetil amonium klorida sebagai molekul pengarah. Modifikasi dilakukan melalui proses dealuminasi, hidrotermal, dan kalsinasi. Dealuminasi dilakukan dengan perendaman dalam larutan HCl 6 M dan NH4NO3 2 M dilanjutkan dengan pemanasan pada temperatur 250 °C. Proses hidrotermal dilakukan di dalam autoklaf pada temperatur 150 °C selama 24 jam dengan penambahan molektil pengarah lauril benzil dimetil amonium klorida pada konsentrasi 10 %, 20 %, 30 % (b/b). Selanjutnya dilakukan kalsinasi pada temperatur 400 °C selama 4 jam. Adsorpsi nitrogen digunakan untuk menentukan distribusi ukuran pori. Uji adsorpsi dilakukan terhadap zeolit termodifikasi menggunakan larutan fenol sebagai adsorbat. Hasil pengukuran distribusi ukuran pori menunjukkan telah terjadi perubahan ukuran pori dari 81,9 % berukuran di bawah 20 A menjadi 100 % berukuran di atas 20 A setelah modifikasi. Kemampuan adsorpsi zeolit hasil modifikasi terhadap senyawa fenol sebesar 0,102 mmol/g pada konsentrasi lauril benzil dimetil amonium klorida 20 %. The pores of natural zeolites had been modified by lauryl benzyl dimethyl ammonium chloride as template molecules. Modification consist of dealumination, hydrothermal, and calcination. Dealumination on natural zeolites had been done by immersing in 6 M hydrochloride acid and 2 M ammonium nitrate solution and calcinated at 250 °C. Hydrothermal reaction was carried out under lauryl benzyl dimethyl ammonium chloride addition with the concentration variation of 10, 20, and 30 % (w/w} at 150 °C for 24 hours and calcinated at 400 °C for 4 hours. The pore characteristics had been analyzed with nitrogen adsorption method. Adsorption test was done using fenol as adsorbate. The pores of natural zeolites changed from 81.9 % below 20 A until 100 % up to 20 A in radius after modification. Adsorption activity for modified zeolite with 20 % lauryl benzyl dimethyl ammonium chloride was 0.102 mmol/g iv
| Item Type: | Thesis (Undergraduate) |
|---|---|
| Subjects: | Q Science > QD Chemistry |
| Divisions: | Faculty of Science and Mathematics > Department of Chemistry |
| ID Code: | 30969 |
| Deposited By: | Mr UPT Perpus 1 |
| Deposited On: | 10 Nov 2011 13:19 |
| Last Modified: | 10 Nov 2011 13:19 |
Repository Staff Only: item control page