Utami , Megasari (2003) Pengendapan logam perak dengan metoda elektrolisis internal. Undergraduate thesis, FMIPA UNDIP.
| PDF Restricted to Repository staff only 1456Kb | ||
| PDF 15Kb | |
| PDF 371Kb | |
| PDF 478Kb | |
| PDF 391Kb | |
| PDF 512Kb | |
| PDF 425Kb | |
| PDF Restricted to Repository staff only 527Kb | ||
| PDF 322Kb | |
| PDF 357Kb | |
| PDF 357Kb | |
| PDF 437Kb |
Abstract
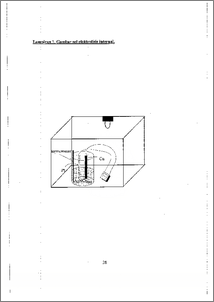
Logam perak bersifat toksik dilingkungan dengan ambang batas 0,05 mg/L. Logam perak dapat diendapkan dengan metoda elektrolisis internal tetapi belum diketahui kondisi pengendapan perak yang dlapat memberikan endapan perak yang banyak. Aplikasi elektrolisis internal pada pengendapan logain perak dilakukan untuk mengetahui kondisi pengendapan perak yaitu waktu dan suhu elektrolisis serta pengaruh penggunaan membran pada anoda. Elektrolisis internal adalah salah satu metoda elektrogravimetri yaitu larutan dengan elektroda tercelup di dalamnya menyusun sebuah sel galvani yang menghasilkan arusnya sendiri. Arus tersebut menyebablcan pengendapan elektrolitik logarn pada katoda. Arus dan tegangan dihasillcan ketika katoda platina dan anoda tembaga dimasukkan ke dalam larutan AgNO3 0,02 N. Elektron yang dihasilkan ditangkap oleh ion-ion perak sehingga mengendap pada katoda. Larutan sisa elektrolisis dianalisis dengan AAS untuk mengetahui konsentrasi perak yang tidak terendapkan. Hash' penelitian menunjukkan waktu dan suhu elektrolisis serta pengaruh penggunaan membran pada anoda. Kondisi pengendapan perak dengan elektrolisis internal efektif dilakukan selama waktu 135 menit, suhu 50 °C dan tanpa penggunaan membran pada anoda. Limbah fotografi merupakan salah satu sumber utama penghasil limbah perak di linglcungan. Kondisi pengendapan perak dengan metoda elektrolisis internal yang diperoleh diterapkan untuk mengurangi kadar perak pada limbah fotografi. Hasil menunjukkan metoda elektrolisis internal dapat menurunkan konsentrasi perak pada limbah fotografi sampai 32,51 %. Silver metal gives toxicity in environmental with maximum contaminant level Q.05 mg/L. Silver could be deposited with internal electrolysis method but the condition of silver deposition that could gives large quantity of silver deposit was unknown. Aplication of internal electrolysis to the deposition of silver was examined to detemine the conditions of silver deposition that are time and electrolysis temperature also coloid membrane effect on anode. Internal electrolysis is one of the methods in electrogravimetry where solution with electrodes immersed in it constitutes a galvanic cell producing its own current. The current causes electrolytic deposition of metal on a cathode. The current and voltage cell were produced as platinum cathode and copper anode immersed in electrolytes containing AgNO3 0.02 N solution. Electrons produced catched by silver ions so that its deposited in cathode. After electrolysis the solution examined with AAS to know silver concentration that did not deposited in cathode. Results showed time and electrolysis temperature also coloid membrane effect on anode. Silver deposition conditions by internal electrolysis were effectively done at 135 minutes, temperature of 50 °C and without membrane. Photographic waste is on of the main source of silver waste in environmental. Silver deposition conditions with internal electrolysis method were used to reduced silver concentration in photographic waste. Results showed that silver concentration decreaSed up to 32.51 %.
| Item Type: | Thesis (Undergraduate) |
|---|---|
| Subjects: | Q Science > QD Chemistry |
| Divisions: | Faculty of Science and Mathematics > Department of Chemistry |
| ID Code: | 30962 |
| Deposited By: | Mr UPT Perpus 1 |
| Deposited On: | 10 Nov 2011 12:49 |
| Last Modified: | 10 Nov 2011 12:49 |
Repository Staff Only: item control page