Purwaningsih , Yuli (2003) Senyawa organoiodium fraksi air turunan glukosa. Undergraduate thesis, FMIPA UNDIP.
| PDF Restricted to Repository staff only 1071Kb | ||
| PDF 14Kb | |
| PDF 349Kb | |
| PDF 446Kb | |
| PDF 384Kb | |
| PDF 467Kb | |
| PDF 357Kb | |
| PDF Restricted to Repository staff only 450Kb | ||
| PDF 317Kb | |
| PDF 345Kb | |
| PDF 319Kb |
Abstract
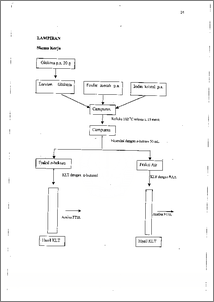
Masyarakat mengkonsumsi iodium dari garam dapur beriodium. Tetapi iodium dalam garam tersebut mullah menghilang karena penyimpana.n. Hal ini menyebabkan iodium dalam garam tersebut menjadi 12. Agar hal ini tidak terjadi maka diusahakan iodium dapat terikat secara kovalen dengan glukosa, yaitu suatu biomolekul yang mudah terserap oleh tubuh. Bahan-bahan kimia yang digunakan dalam penelitian ini adalah glukosa, iodin dan fosfor merah di dalam set alat refluks pada suhu 102 °C selama ± 15 menit. Setelah itu, campuran diekstraksi menggunakan n-heksan dan fraksi air dianalisis menggunakan metode KLT dengan pengembang n-butanol : asam asetat : air (B : A : A) dengan perbandingan 4 : 1 : 5. Dan metode tersebut dapat diketahui bahwa fraksi air mengandung senyawa organik yang mempunyai Rf 0,65. Selanjutnya dianalisis menggunakan spektrofotometer FTIR yang menunjukkan vibrasi C-1 pada daerah 617,2. cm -1. Hal ini menunjukkan bahwa senyawa organoiodium terbentuk dalam pelarut air. Consumable iodine-desk salt have being accuring significantly iodine lossing for disposal. It was caused iodine easy to be 12 volatile in the salt. In order to prevent those the iodine should be covalently bonded with glucose , which is a biomolecule absorbed easily by human-metabolism process. The chemicals used in this research were glucose, iodine and red phosphorus. These chemicals were reacted in reflux set on 102 °C for ± 15 minutes. After that, it was then extracted with n-hexane and water fraction was analyzed by TLC methods with development solvent of n-Butanol : Acetic acid : Water (B : A : W) with 4 : 1 : 5 ratio. From this method could be determined the fraction contained an organic substance which has Rf of 0.65. Further analysis employing FT1R spectrophotometer indicated that C-1 vibration at 617.2 cm'. It was indicated that an organoiodium could be formed in water solution.
| Item Type: | Thesis (Undergraduate) |
|---|---|
| Subjects: | Q Science > QD Chemistry |
| Divisions: | Faculty of Science and Mathematics > Department of Chemistry |
| ID Code: | 30918 |
| Deposited By: | Mr UPT Perpus 1 |
| Deposited On: | 09 Nov 2011 15:28 |
| Last Modified: | 09 Nov 2011 15:28 |
Repository Staff Only: item control page