Azis , Dwi Yulianto (2002) Pemanfaatan kitosan dari limbah udang (Penaeus merguensis) sebagai adsorben ion logam nikel. Undergraduate thesis, FMIPA UNDIP.
| PDF Restricted to Repository staff only 1509Kb | ||
| PDF 14Kb | |
| PDF 355Kb | |
| PDF 478Kb | |
| PDF 354Kb | |
| PDF 617Kb | |
| PDF 412Kb | |
| PDF Restricted to Repository staff only 520Kb | ||
| PDF 321Kb | |
| PDF 356Kb | |
| PDF 459Kb |
Abstract
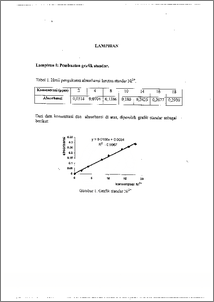
Industri pengolahan udang di Indonesia telah menghasilkan limbah yang cukup besar jumlahnya tetapi belum diikuti dengan pemanfaatannya secara maksimal. Hasil penelitian menunjukkan bahwa limbah yang berupa kepala dan kulit udang mengandung senyawa kitin yang dapat diproses lebih lanjut menjadi kitosan. Kitin dapat diisolasi melalui proses deproteinasi, demineralisasi dan depigmentasi. Kitosan diperoleh dari transformasi kitin melalui proses deasetilasi. Kitosan mempunyai gugus amina yang dapat berfungsi sebagai ligan untuk membentuk ikatan kovalen dengan ion logam transisi dan ion logam berat. Pada penelitian ini kitosan yang diperoleh dimanfaatkan sebagai adsorben ion logam nikel. Dan tahap isolasi diperoleh 18,92 % kitin dan hasil transformasi diperoleh 7,18 % kitosan dengan kadar air 11,04 %, kadar abu 1,12 % dan derajat deasetilasi 50,44 %. Adsorpsi ion Nit + menggunakan kitosan dilakukan pada variasi pH = 4; 4,5; 5; 5,5 dan 6, kecepatan pengadukan 150 rpm, waktu kontak 2 jam dan berat kitosan 0,5 g. Hasil analisis menggunakan AAS diperoleh iaformasi bahwa pada pH = 5 kitosan mempunyai kapasitas adsorpsi optimum sebesar 13,1x10-2 mg/g. Shrimp processing industry in Indonesia has produced waste in large quantities but its utilization was not complete. The research result show that shrimp waste containing chitin which can processed into chitosan. Chitin can be isolated from shrimp waste by deproteination, demineralization and decoloration. Chitosan was produced by transforming chitin through deacetylation. Chitosan has the amine groups functioning as ligands to form covalen coordination bonds with heavy metal and transition metal ions. In this research chitosan was used as an adsorben for Nit' ion. From the isolation process was obtained 18,92 % of chitin and its transformation yield 7.18 % of chitosan having 11.04 % of water, 1.12 % of ash and 50.44 % deacetylation. Adsorption of Nit + ion using chitosan was carried out at pH variation of 4; 4.5; 5; 5.5 and 6, while the stirring speed (150 rpm), contact time (2 hours) and the amount of chitosan (0.5 g) were kept constant. Based on the analysis results, it was known that chitosan has optimum adsorption capacity at pH = 5 (13.1x10-2 mg/g).
| Item Type: | Thesis (Undergraduate) |
|---|---|
| Subjects: | Q Science > QD Chemistry |
| Divisions: | Faculty of Science and Mathematics > Department of Chemistry |
| ID Code: | 30895 |
| Deposited By: | Mr UPT Perpus 1 |
| Deposited On: | 08 Nov 2011 15:01 |
| Last Modified: | 08 Nov 2011 15:01 |
Repository Staff Only: item control page