Bahri , Ikhsanusin (2005) Pengambilan kembali Surfaktan dari model limbah Cair cucian menggunakan metode sublasi. Undergraduate thesis, FMIPA UNDIP.
| PDF Restricted to Repository staff only 1509Kb | ||
| PDF 15Kb | |
| PDF 380Kb | |
| PDF 465Kb | |
| PDF 412Kb | |
| PDF 554Kb | |
| PDF 412Kb | |
| PDF Restricted to Repository staff only 475Kb | ||
| PDF 346Kb | |
| PDF 358Kb | |
| PDF 473Kb |
Abstract
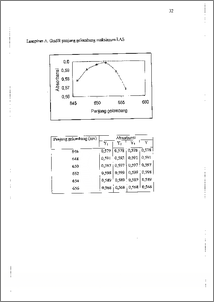
Proses sublasi surfaktan dari model limbah cair cucian telah dilakukan untuk mempelajari pengaruh partikel kotoran terhadap jumlah surfaktan yang dapat terambil kembali. Hal tersebut dilakukan mengingat surfaktan yang terbuang ke perairan dapat bercampur dengan kotoran lainnya sehingga hasil dari proses sublasi surfaktan pada limbah cair akan berbeda dengan hasil dari proses sublasi pada larutan surfaktan murni. Sublasi adalah metode pengambilan kembali surfaktan dari larutannya yang berdasar pada adsorbsi antar muka gas-cair. Metode tersebut terbukti efektif dengan recovery 98% dan hasil isolat yang relatif murni. Proses sublasi dilakukan dengan mengalirkan gas nitrogen ke dalam tabung sublator yang mengandung surfaktan dan lapisan etil asetat pada bagian permukaannya. Surfaktan teradsorbsi pada antar muka gas-cair yang berwujud gelembung-gelembung dan dibawa ke lapisan etil asetat. Gelembung-gelembung keluar menuju udara meninggalkan larutan sampel dan surfaktan terlarut dalam etil asetat. Larutan surfaktan dalam etil asetat dipisahkan dan divapkan, surfaktan yang tersisa dianalisa. Proses sublasi dilakukan dengan penambahan garam dan tanpa penambahan garam. Garam yang ditambahkan adalah NaCl dan NaHCO3 pada komposisi masing-masing 80 gram dan 4 gram. Analisa hasil sublasi menggunakan metode MBAS dengan alai spektrometer UV-Vis dan spektrometer FTIR. Spektra FTIR dari hasil sublasi dan dari senyawa murni memiliki kesamaan gugus-gugus fungsi. Recovery surfaktan pada larutan detergen A, B, C tanpa penambahan garam sebesar 24,24%; 28,57%; 26.34%, sedangkan untuk model limbahnya sebesar 22,33%; 26,31%; 19,75%. Recovery sublasi pada larutan detergen dengan penambahan garam adalah 82,29%; 83,65%; 79,54% sedangkan untuk model limbahnya adalah 80,58%; 78,36%; 80,58%. Data tersebut dapat disimpulkan bahwa metode sublasi mampu mengambil kembali surfaktan dari larutan model limbah. Terdapatnya partikulat pada model limbah cenderung menurunkan hasil sublasi. Recovery surfactant from waste water model by sublation method has been done to study correlation particulates with percent recovery sublation result. We studied its because surfactant and particulates can mixture, so percent recovery at waste water make different with pure surfactant solution. Sublation is a method to recover surfactant from its solution which is based on adsorption at gas-aqueous interfaces. It is accomplished by bubbling a stream of nitrogen up through a sponge and column containing the sample and an overlying of ethyl acetate. The surfactant is adsorbed at the water-gas interfaces of the bubbles and is carried into the ethyl acetate layer. The bubbles escape into the atmosphere leaving behind the surfactant dissolved in ethyl acetate. The solvent is separated and evaporated leaving surfactant as a residue for analysis. Sublation process had been done for pure sample and waste model by additional salt to the surfactant solution. The salts are NaC1 and NaHCO3 by 80 grams and 4 grams composition. Sublation result analysed with MBAS method by UV-Vis Spectrometer. Identification of groups by FTIR Spectrometer. FTIR spectrum both pure surfactant and sublation, result had same groups. Recovery of surfactant from its non-salt solution was 24.24%, 28.57%, 26.34%, for A, B, C pure samples respectively, and 22.33%, 26.31%, 19.75% respectively for A, B, C waste model. And recovery by additional salt was 82.29%, 83.65%, 79.54% for A, B, C pure samples respectively. While recovery for A, B, C waste models were 80.58%, 78.36%, 80.58% respectively. We get conclusion that sublation method can recover surfactant from waste water model and particulate present tend to reduce percent recovery sublation result.
| Item Type: | Thesis (Undergraduate) |
|---|---|
| Subjects: | Q Science > QD Chemistry |
| Divisions: | Faculty of Science and Mathematics > Department of Chemistry |
| ID Code: | 30866 |
| Deposited By: | Mr UPT Perpus 1 |
| Deposited On: | 08 Nov 2011 10:09 |
| Last Modified: | 08 Nov 2011 10:09 |
Repository Staff Only: item control page