Hastari , Rina (2002) Penentuan laju korosi logam zirkaloy-4 dalam air laut. Undergraduate thesis, FMIPA UNDIP.
| PDF Restricted to Repository staff only 1370Kb | ||
| PDF 15Kb | |
| PDF 353Kb | |
| PDF 457Kb | |
| PDF 367Kb | |
| PDF 565Kb | |
| PDF 401Kb | |
| PDF Restricted to Repository staff only 488Kb | ||
| PDF 325Kb | |
| PDF 368Kb | |
| PDF 411Kb |
Abstract
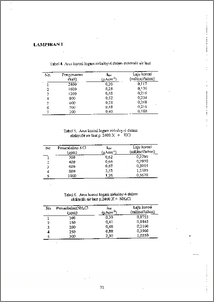
Zirkaloy-4 merupakan logam paduan antara logam zirkonium dengan logam-logam lainnya, yang digunakan sebagai bahan konstruksi pipa pendingin pada reaktor nuklir, meskipun zirkonium sangat elektronegatif (E° = -1,529 V) namun memiliki ketahanan yang cukup baik dalam lingkungan air dengan terbentuknya lapisan pasif pada pennukaan logam. Analisis dilakukan berdasarkan persamaan Tafel menggunakan alat potensiostat. Daft persamaan ini akan diperoleh I. Preparasi dilakukan dengan variasi konsentrasi air laut dan diikuti dengan penambahan KCI, NH4C1 dan HC1 pada konsentrasi air laut terendah (pengenceran 2400 kali) dengan konsentrasi awal sebesar 19.830 ppm.. Eksperiment dilakukan dari potensial awal —2000 mV dan potensial akhir 2000 mV, dengan laju scan 20 mV/min. Dari percobaan diperoleh basil anus korosi tinggi logam zirkaloy-4 dalam air laut pada pengenceran 600 kali dengan Ikor = 0) = 0,58 i.tAcm-2, penambahan KCI 800 ppm Ikor (i 0) = 2,21 pAcm-2, penambahan NFI4C1 300 ppm Ikor (i = 0) = 2,53 pAcm-2, penambahan HCI 400 ppm Ik„, (1 = 0) = 2,3 ptAcm—. Zirconium and it's alloys are finding increasing applications especially in water-cooled nuclear reactors. Although zirconium is very electronegative (E° = -1.529 V), it is highly corrosion resistant to aqueous media. This corrosion resistance results from the formation of protectie, strongly adherent passive films on the metal surface. The corrosion experiment has been observed in Tafel equation with potensiostat. Voi, could be calculated from this equation. Experiment was done by varying the concentration of sea water and the addition of HCI, NH4C1 and HCl to sea water with low concentration. The concentration of chloride ion in sea water was 19.830 ppm. Experiment were done under starting potensial of-2000 mV and ending potensial of 2000 mV with scanning rate of 20 mV/min, for each samples. It was found that corrosion current of zircaloy-4 were lc„, (i = 0) = 0,58 gAcm-2, in sea water with concentration at 600 time dilute, Te,„,, (i = 0) = 2,2 AAcm-2, in KCl 800 pprn, I (i = 0) = 2,53 gAcm-2 in NH4C1 300 ppm, Ic„, (i = 0) = 2,3 f.tAcm-2 in HCI 400 ppm.
| Item Type: | Thesis (Undergraduate) |
|---|---|
| Subjects: | Q Science > QD Chemistry |
| Divisions: | Faculty of Science and Mathematics > Department of Chemistry |
| ID Code: | 30856 |
| Deposited By: | Mr UPT Perpus 1 |
| Deposited On: | 08 Nov 2011 08:44 |
| Last Modified: | 08 Nov 2011 08:44 |
Repository Staff Only: item control page