Hasanah , Herlina Wati (2002) Eksplorasi dan identifikasi metabolit sekunder dari daun srikaya (Annona squamosa). Undergraduate thesis, FMIPA UNDIP.
| PDF Restricted to Repository staff only 1350Kb | ||
| PDF 16Kb | |
| PDF 348Kb | |
| PDF 480Kb | |
| PDF 398Kb | |
| PDF 545Kb | |
| PDF 395Kb | |
| PDF Restricted to Repository staff only 518Kb | ||
| PDF 330Kb | |
| PDF 372Kb | |
| PDF 324Kb |
Abstract
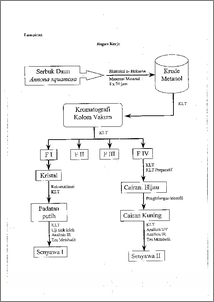
I)alam rangka memperkaya profil kimia tanaman srikaya (Annona squamosa) telah dilakukan eksplorasi metabolit-metabolit sekunder yang terkandung di dalam daunnya. Pemisahan dilakukan dengan kromatograft kolom yak= menggunakan fasa gerak n-helcsana—etilasetat dengan gradien kepolaran dan fasa diamnya silika gel. Dihasilkan senyawa I berbentuk padatan putih dan senyawa II berbentuk cairan kuning. Senyawa tersebut dianalisis dengan alat spektroskopi IR, UV, uji kimia dan uji titik leleh. Senyawa I adalah triterpena yang mempunyai titik leleh 73 — 75 °C, dan analisis data IR menunjukkan gugus hiroksil terikat, diena terkonjugasi dan gugus metilen. Sedangkan senyawa II mengandung komponen mayor senyawa alkaloid dengan gugus fenol, diena tak terkonjugasi pada inti benzena, N siklik serta menyerap pada panjang gelombang 206 nm. Sedangkan struktur Iengkap senyawa dan II belum dapat ditentukan. In behalf enrich chemical profile of sugar apple (Annona squamosa) have been carried out exploration of secondary metabolites included in the sugar apple leave.The separation was applied to vacuum coloumn chromagraphy with mobile phase of n-hexane—ethyl acetate with polarity of gradient and stationary phase of silica gel. The research was resulted white crystalline and yellow liquid (first and second compound). Those compounds were analized by IR, UV spectroscopy, chemical and melting point test. The first compound was triterpenoid that had melting ponit at 73 — 75 °C. Based on interpretation of IR data was shown that compound I had bonded hidroksil, conjugation of diena and methylen substituents. The second compound included major component was phenolic alkaloid with disconjugation of diena in benzene core, N cyclic and so was absorbed wavelength at 206 rim
| Item Type: | Thesis (Undergraduate) |
|---|---|
| Subjects: | Q Science > QD Chemistry |
| Divisions: | Faculty of Science and Mathematics > Department of Chemistry |
| ID Code: | 30844 |
| Deposited By: | Mr UPT Perpus 1 |
| Deposited On: | 08 Nov 2011 07:46 |
| Last Modified: | 08 Nov 2011 07:53 |
Repository Staff Only: item control page