Wahyono , Agus (2001) Peranan Trifenilfosfin dalam Dekuarterneerisasi senyawa Diisopropil Dimetil Amonium Iodida. Undergraduate thesis, FMIPA UNDIP.
| PDF Restricted to Repository staff only 1361Kb | ||
| PDF 16Kb | |
| PDF 345Kb | |
| PDF 443Kb | |
| PDF 423Kb | |
| PDF 586Kb | |
| PDF 372Kb | |
| PDF Restricted to Repository staff only 521Kb | ||
| PDF 333Kb | |
| PDF 368Kb | |
| PDF 333Kb |
Abstract
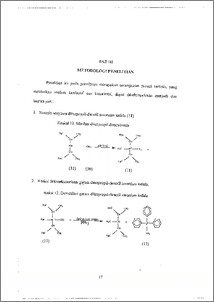
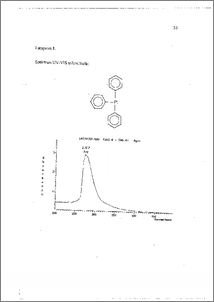
Transformasi amina tersier dilakukan untuk inemperoleh senyawa turunannya yang lebih potensial. Sahli satu tahap penting dalam transformasinya adalah dek.uarternerisasi spesifik garain aiTiOniurn yang disintesis dari alkilasi terhadap amina primer atau sekunder. Penggunaan nukleofil eksternal yang tepat sangat berpengaruh dal= reaksi dekuarternerisasi. Garam diisopropil-dimetil amonium iodida dapat didekuarternerisasi dengan adinya trifenilfosfin (PPh3) dalam pelarut dimetil formamida (DMF). Analisis produk dengan spektrorneter UV-V1S dan ineminjukkan terjadinya reaksi demetilasi dalam reaksi dekuarternerisasi selama 18 jam dengan rendernen sebesar 85 %. Hal ini menunjukkan bahwa PPh3 dapat merangsang reaksi dekuarternerisasi spesifik terhadap elektrofil dengan halangan sterik rendah dari garam amonium, terutama garam diisopropii-dimetil amonium iodida. Tertiary amine transformation was done to get its potential derivates. One of its important step of transformations is specific dequaternarisation of ammonium salt synthesized by alkylations of • primary or • -secondary. amine. Using external nucleophyle appropriately is very influential in dequaternarisation. Diisoprophyl¬dimethyl ammonium iodide salt was able to be dequaternarised by triphenylphosphine (PP113)‘ in DMF reflux. Analyzing product with Spectrometer UV-VIS and 11-1-NMR showed that demethylation was occurred during •18 hours dequaternarisation with 85 % in yields. Its showed that PPh3 stimulate specific dequaternarisation to the small steric hindrance electrophyle of aliphatic ammonium salt, especially diisoprophyl-dirnethyl ammonium iodide. This docunient is Undip Institutional Repository Collection. The author(s) o 146 submission to any medium or format for the purpose of preservation. The author(s, submission for purposes of security, back-up and preservation. ( http://eprints.undip.
| Item Type: | Thesis (Undergraduate) |
|---|---|
| Subjects: | Q Science > QD Chemistry |
| Divisions: | Faculty of Science and Mathematics > Department of Chemistry |
| ID Code: | 30832 |
| Deposited By: | Mr UPT Perpus 1 |
| Deposited On: | 07 Nov 2011 15:06 |
| Last Modified: | 07 Nov 2011 15:06 |
Repository Staff Only: item control page