Anjarwati , Dhiah (2001) Isolasi, Fraksinasi, dan penentuan aktivitas spesifik Asparaginase dari Asparagus (Asparagusofficinalis). Undergraduate thesis, FMIPA UNDIP.
| PDF Restricted to Repository staff only 1701Kb | ||
| PDF 15Kb | |
| PDF 369Kb | |
| PDF 478Kb | |
| PDF 412Kb | |
| PDF 568Kb | |
| PDF Restricted to Repository staff only 451Kb | ||
| PDF 520Kb | |
| PDF 333Kb | |
| PDF 603Kb |
Abstract
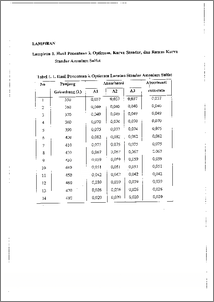
Asparaginase merupakan enzim yang termasuk golongan hidrolase yang dapat menguraikan asparagin menjadi asam aspartat dan amonia dengan memutuskan ikatan amida. Enzim ini menyebabkan pengurangan secara cepat asparagin serum yang diperlukan untuk pertumbuhan kanker tertentu, sehingga pertumbuhan kanker tersebut terhambat. Dari hal tersebut, maka dilakukan penelitian untuk mengisolasi dan mengkarakterisasi enzim asparaginase dari Asparagus officinalis. Isolasi dilakukan dengan metode ekstraksi, hasil isolasi difraksinasi bertingkat menggunakan amonium sulfat dan dilanjutkan dialisis dengan buffer Tris. Penelitian dilakukan dengan memvariasi pH, temperatur dan waktu inkubasi untuk menentukan kondisi optimum enzim asparaginase dalam menghidrolisis substratnya. Penentuan aktivitas enzim menggunakan metode Nessler dan penentuan kadar protein menggunakan metode Lowry. Hasil Penelitian menunjukkan bahwa aktivitas spesifik enzim pada Fl, F2, F3, F4 dan F5 berturut-turut adalah 0,2820 U.mg protein-1, 2,2134 U.mg protein-I, 13,5024 U.mg protein', 10,1253 U.mg protein-I, 18,8588 U.mg protein-I dan 28,0483 U.mg protein-I. Berdasarkan hasil penelitian dapat disirnpulkan bahwa Fraksi 5 ( F5 dengan tingkat kejenuhan 80-100 %) memiliki tingkat kemumian yang tertinggi dengan aktivitas spesifik sebesar 28,0483 U.mg proteirC1. Dan berdasarkan penentuan sifat karakteristiknya, enzim asparaginase dari Asparagus officinalis ini bekerja optimal pada pH 8,0, temperatur 37 dan waktu inkubasi 30-menit. Asparaginase is one of hydrolitic enzyme which is capable of decomposing asparagine into aspartic acid and ammonia by breaking amide bond This enzyme quickly reduces asparagine serum which is needed by certain cancer to grow so that the cancer growth is impeded. Based on this, a research has been conducted to isolated and characterize asparaginase enzyme from Asparagus officinalis. Isolation was done through extraction. The isolate was fractionized in stages using ammonium sulphate and was then analyzed with Tris buffer. The research was conducted by varying the pH, temperature, and incubation time to determine the optimum condition of asparagine enzyme in hydrolizing its substrate. The determination of enzyme activity was performed with Nessler method while the determination of protein content was performed with Lowry method. The result of the research showed that specific activity of the enzyme at F1, F2, F3, F4, and F5 were 0.2820 U.mg protein-I, 2.2134 U.mg protein-1, 13.5024 U.mg protein-1, 10.1253 U.mg protein-I, 18.8588 U.mg protein-I and 28.0483 U.mg protein-I respectively. The result led to the conclusion that fraction 5 (F5 with saturation level of 80-100 %) possessed the highest purity level with specific activity of 28.0483 U.mg protein-I. Based upon the determination of its characteristics asparaginase enzyme from Asparagus 6fficinalis works optimally at pH 8.0, temperature 37 °C and incubation time of 30 minutes.
| Item Type: | Thesis (Undergraduate) |
|---|---|
| Subjects: | Q Science > QD Chemistry |
| Divisions: | Faculty of Science and Mathematics > Department of Chemistry |
| ID Code: | 30818 |
| Deposited By: | Mr UPT Perpus 1 |
| Deposited On: | 07 Nov 2011 14:09 |
| Last Modified: | 07 Nov 2011 14:09 |
Repository Staff Only: item control page