Hamdani , Asep (2001) Pengaruh kandungan logam aktif terhadap luas permukaan dan keasaman dalam pembuatan Katalis Mo-Co/∂-Al₂0₃. Undergraduate thesis, FMIPA UNDIP.
| PDF Restricted to Repository staff only 1775Kb | ||
| PDF 17Kb | |
| PDF 356Kb | |
| PDF 470Kb | |
| PDF 398Kb | |
| PDF 725Kb | |
| PDF 445Kb | |
| PDF Restricted to Repository staff only 545Kb | ||
| PDF 328Kb | |
| PDF 369Kb | |
| PDF 502Kb |
Abstract
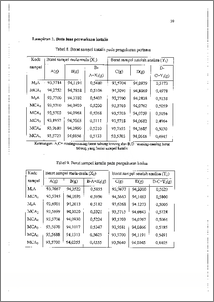
Telah dilakukan penelitian pembuatan katalis Mo-Co/T-Al203 dengan metode impregnasi basah bertahap, dilanjutkan pengeringan dan kalsinasi. Konsentrasi awal logam Mo dan logam Co dibuat bervariasi, sedangkan waktu impregnasi, berat penyangga, serta kondisi pengeringan dan kalsinasi dibuat tetap. Karakterisasi katalis meliputi penentuan luas pennukaan total dengan metode adsorpsi gas nitrogen, penentuan keasaman katalis dilakukan secara gravimetri dan spektroskopi FTIR. Adapun kandungan logam katalis ditentukan menggunakan AAS. Hasil analisa AAS menunjukkan bahwa preparasi katalis dengan komposisi mula-mula Mo sebesar 15,7385 % dan Co 2,9356 % b/v menghasilkan katalis dengan kandungan MoO3 sebesar 13,1435 % dan CoO 2,2884 % b/b adalah katalis dengan kandungan logam aktif optimum. Luas permukaan total katalis dan keasaman katalis cenderung menurun dengan semakin meningkatnya kandungan total logam oksida. Pada spektra FTIR menunjukkan bahwa katalis mampu mengadsorpsi piridin pada bilangan gelombang 1447,50 cm-1 yang mengindikasikan adanya situs asam Lewis, clan. pada bilangan gelombang 1649,96 cm-1 adalah karakteristik untuk jenis asam Bronsted. Preparation of Mo-Copy-Al203 catalyst has been carried out by successive wet-impregnation methode and followed by drying and calcination. The preparation variabels were both of metal precursor concentration whereas time of impregnation, support weight, drying and calcinating condition were made constant. The characterisation included total surface area measurment by nitrogen gas adsorption methode, acidity of catalyst with gravimetry determining and FT[R spectroscopy, while metal contents catalyst determining by AAS. The results of AAS showed that catalyst preparation by using composition of precursor Mo amount to 15.7385 % and Co 2.9356 % w/v gave the catalyst, with the composition of MoO3 amount to 13.1435 % and Co 2.2884 % w/w were the catalyst optimum metals composition. Result of catalyst surface area and acidity measunnent tend to decrease proportional to the raising of total metal oxide contents. Spectrum FTIR of catalyst which pyridin adsorptions were appeared at 1447.50 and 1649.96 cm-I, the peak of wave number. It was the characteristics of Lewis and Bronsted acid.
| Item Type: | Thesis (Undergraduate) |
|---|---|
| Subjects: | Q Science > QC Physics |
| Divisions: | Faculty of Science and Mathematics > Department of Chemistry |
| ID Code: | 30810 |
| Deposited By: | Mr UPT Perpus 1 |
| Deposited On: | 07 Nov 2011 13:00 |
| Last Modified: | 07 Nov 2011 13:00 |
Repository Staff Only: item control page