Suhari , Muh (2001) Isolasi dan karakterisasi Enzim - Amilase dari Ubi jalar(Ipomoea batatas L). Undergraduate thesis, FMIPA UNDIP.
| PDF Restricted to Repository staff only 1601Kb | ||
| PDF 14Kb | |
| PDF 348Kb | |
| PDF 419Kb | |
| PDF 345Kb | |
| PDF 609Kb | |
| PDF 445Kb | |
| PDF Restricted to Repository staff only 543Kb | ||
| PDF 321Kb | |
| PDF 343Kb | |
| PDF 590Kb |
Abstract
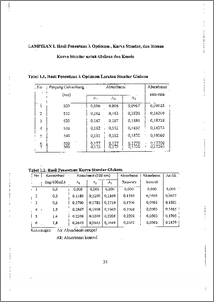
Enzim a-amilase dapat mengubah amilum menjadi glukosa dengan merusak ikatan a-1,4 glikosida dan diuhah menjadi glukosa. Telah dilakukan isolasi dan karakterisasi enzim a-amilase dari ubi jalar dengan menggunakan substrat amilum. Basil isolasi difraksinasi secara bertingkat dengan (NH4)2SO4 yaitu F1 (0-10 %), F2 (10-30 %), F3 (30-50 %), F4 (50-70 %), F5 (70-100 %) dan dilanjutkan dengan dialisis. Produk glukosa dianalisa dengan Metode Nelson-Somougyi dan kadar protein ditentukan dengan metode Lowry yang dinyatakan dalam bentuk unit aktivitas dimana produk yang terbentuk merupakan hasil aktivitas enzim a-amilase terhadap substrat pada kondisi optimal. Hasil penelitian menunjukkan bahwa F3 memiliki aktivitas spesifik yang terbesar 11,8662 U/mg, karakterisasi pada pH optimum 6,1 suhu optimum 65 °C dan waktu inkubasi optimum 30 menit dengan aktivitas spesifik berturut-turut sebagai berikut:15,8317 ; 15, 1657 ; 15,8811 U/mg. a-amylase is an enzyme which can change amylum by breaking a-1,4 glycoside bond into monomer glucose. Isolation and characterization of the a—amylase enzyme from sweet potatoes has been conducted with arnylum substrate. The result of isolation was fractioned with (NH4)2SO4 in stages which were F1 (0-10 %), F2 (10-30 %), F3 (30-50 %), F4 (50-70 %), F5 (70-100 %) and continued with dialysis. The glucose produced was analyzed with Nelson-Somougyi method by using spectrophotometer UV-Vis and concentration of enzyme was calculated by Lowry method., which glucose was produced by a-amylase activity to substrate at optimum condition. Reseach showed that fraction 3 (30-50 %) had the biggest specific activity of 11.8662 U/mg, characterization at optimum pH — 6,1 , optimum temperature 65 °C and optimum incubation time 30 minutes showed specific activity of: 15.8317; 15.1657; 15.8811 U/mg respectively.
| Item Type: | Thesis (Undergraduate) |
|---|---|
| Subjects: | Q Science > QD Chemistry |
| Divisions: | Faculty of Science and Mathematics > Department of Chemistry |
| ID Code: | 30808 |
| Deposited By: | Mr UPT Perpus 1 |
| Deposited On: | 07 Nov 2011 12:51 |
| Last Modified: | 07 Nov 2011 12:51 |
Repository Staff Only: item control page