Yolanda , Dahlia (1999) Preparasi Karakterisasi dan uji aktivitas Katalis Ni-Mo/y-A1203. Undergraduate thesis, FMIPA UNDIP.
| PDF Restricted to Repository staff only 1576Kb | ||
| PDF 15Kb | |
| PDF 358Kb | |
| PDF 461Kb | |
| PDF 377Kb | |
| PDF 622Kb | |
| PDF 438Kb | |
| PDF Restricted to Repository staff only 608Kb | ||
| PDF 327Kb | |
| PDF 363Kb | |
| PDF 387Kb |
Abstract
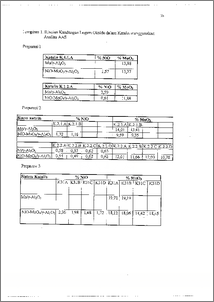
Preparasi katalis telah dilakukan dengan metode impregnasi basah bertahap yang dilanjutkan dengan pengeringan dan kalsinasi. Variabel preparasi yang ditinjau adalah konsentrasi kedua larutan garam prekursor dan pH larutan garam prekursor Ni, karakterisasi katalis meliputi analisa kandungan logam menggunakan AAS dan luas permukaan total menggunakan alat Micromeritic Surface Area Analyzer. Aktivitas katalis diuji dengan alat catatest pada variasi suhu 300, 330 dan 360 °C. Hasil analisis AAS menunjukkan bahwa preparasi katalis dengan konsentrasi larutan garam prekursor Mo 0,0967 M dan Ni 0,2677 M menghasilkan katalis dengan kandungan MoO3 12,58 % dan NiO 1,09 %, sedangkan untuk konsentrasi larutan Mo 0,2031 M dan larutan Ni 0,6800 M menghasilkan katalis dengan kandungan MoO3 17,26 % dan NiO 1,89 %. Perlakuan pH = 7 pada larutan garam prekursor Ni memberikan hasil katalis dengan kandungan logam oksida yang lebih besar daripada pH = 10. Luas permukaan total katalis menunjukan kecenderungan penurunan dengan semakin bertambahnya kandungan logam oksida dalam katalis. Aktivitas katalis bertambah dengan naiknya kandungan logam katalis dan pada suhu 360 °C memberikan basil terbaik dengan konversi 27,43 % Preparation of Ni-MoPy-Al203 catalyst has been carried out by successive wet-impregnation method and then was followed by drying and calcination. The preparation variables were both of precursor solution concentrations and pH of nikel precursor solution, the characterization included metal composition by AAS and total surface area was measured by Micromeritic Surface Area Analyzer. Catalyst activity was tested by using catatest unit as temperature function, respectively at 300, 330 and 360 °C. The result of AAS showed that catalyst preparation by using concentration of Mo salt solution 0,0967 M and Ni 0.2677 M gave result the catalyst with the composition of MoO3 12.58 % and NiO 1.09 %, while concentration of Mo salt solution 0.2031 M and Ni 0.6800 M gave result the catalyst with the composition of MoO3 17.26 % and NiO 1.89 %. pH = 7 on the nikel precurSor solution gave the result of catalyst metal oxide composition higher than pH = 10. Total surface area showed the decline tendency if composition metal oxide was added. Catalyst activity increased as the catalyst metal oxide composition increased too. At 360 °C catalyst activity gave the best convertion 27.43 %.
| Item Type: | Thesis (Undergraduate) |
|---|---|
| Subjects: | Q Science > QD Chemistry |
| Divisions: | Faculty of Science and Mathematics > Department of Chemistry |
| ID Code: | 30751 |
| Deposited By: | Mr UPT Perpus 1 |
| Deposited On: | 07 Nov 2011 07:56 |
| Last Modified: | 07 Nov 2011 07:56 |
Repository Staff Only: item control page