Zaenuri , Agus (1999) Selektifitas Adsorpsi Ion CI dan S0₄ = oleh Zeolit Aktif. Undergraduate thesis, FMIPA Undip.
| PDF Restricted to Repository staff only 1121Kb | ||
| PDF 15Kb | |
| PDF 352Kb | |
| PDF 409Kb | |
| PDF 352Kb | |
| PDF 497Kb | |
| PDF 428Kb | |
| PDF Restricted to Repository staff only 426Kb | ||
| PDF 328Kb | |
| PDF 341Kb | |
| PDF 350Kb |
Abstract
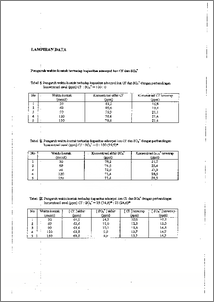
Penelitian ini bertujuan untuk mempelajari selektifitas adsorpsi ion Cr dan SO4= oleh zeolit aktif Zeolit aktif diperoleh dengin cara merendam zeolit dalam asam dan dipanaskan, dilanjutkan dengan perendaman dalam amonium nitrat dan dikalsinasi pada temperatur 450, 500, 550 dan 600 °C, kemudian zeolit aktif digunakan sebagai adsorben dalam proses adsorpsi ion C1- dan Sar. Hasil penelitian menunjukkan temperatur kalsinasi optimum modifikasi zeolit adalah 550 °C. Hal ini ditunjukkan pada adsorpsi dengan konsentrasi awal 100 ppm terserap 19,4 ppm dan 804= dengan konsentrasi awal 99,9 ppm terserap 25,4 ppm. Sedangkan dalam proses adsorpsi, dmi masing-masing perbandingan Cl : SO4 (100 : 0 ; 75 : 25 ; 50 : 50 ; 25 : 75 ; 0 : 100) ppm diperoleh waktu kontak optimum 90 menit. Hasil yang diperoleh menunjukkan bahwa ion SO4= lebih banyak terserap dari pada ion Cl". Dari hasil penelitian ini dapat disimpulkan bahwa ada pengaruh muatan anion pada proses adsorpsi oleh zeolit aktif dan zeolit aktif lebih selektif terhadap ion SO4' dari pada ion Cl". The aim of this research is to study the adsorption selectivity of Cl- and 304' by activated zeolite. The activated zeolite was gotten by bathing zeolite in acid and heated. Next, it was bathed in NH4NO3 and calcinated on temperatur 450, 500, 550 and 600 'C, then activated zeolite is used as the adsorbent at adsorption process of ion Cl- and 304. The result of this research indicate that the optimum calcinating temperature was 550 °C. In this condition ion Cr with the initial concentration 100 ppm was adsorbed 19.4 ppm and 304--' with the initial concentration 99.9 ppm was adsorbed 25.4 ppm. In the other side the optimum contact time in adsorption process was 90 minute with ratio Cl- and 304"-- ( 100: 0 75 : 25 , 50 :50 , 25 : 75 , 0 : 100 ) ppm. It was also indicate ion 304' was more adsorbed than ion Cl". The result of this research indicate that there are influence of anion charge in adsorption process and the activated zeolite is more selective for ion SO4= than ion Cl". This document is Undip Institutional Repository Collection. The author(s) orVopyright owners) agree that UNDIP-IR may, without changing the content, translate the I F i submission to any medium or format for the purpose of preservation. The author(s) or copyright owner(s) also agree that UNDIP-IR may keep more than one copy Of this submission for purposes of security, back-up and preservation. ( http://eprints.undip.ac.id)
| Item Type: | Thesis (Undergraduate) |
|---|---|
| Subjects: | Q Science > QD Chemistry |
| Divisions: | Faculty of Science and Mathematics > Department of Chemistry |
| ID Code: | 30734 |
| Deposited By: | Mr UPT Perpus 1 |
| Deposited On: | 05 Nov 2011 11:02 |
| Last Modified: | 05 Nov 2011 11:02 |
Repository Staff Only: item control page