Hidayat , Yohan (1998) Pemanfaatan Peroksidase ari lobak sebagai katalis untuk penanganan limbah fenol dengan Hidrogen Feroksida. Undergraduate thesis, FMIPA UNDIP.
| PDF Restricted to Repository staff only 2223Kb | ||
| PDF 16Kb | |
| PDF 346Kb | |
| PDF 419Kb | |
| PDF 354Kb | |
| PDF 688Kb | |
| PDF 460Kb | |
| PDF Restricted to Repository staff only 851Kb | ||
| PDF 323Kb | |
| PDF 356Kb | |
| PDF 785Kb |
Abstract
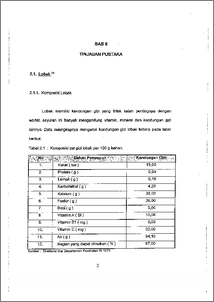
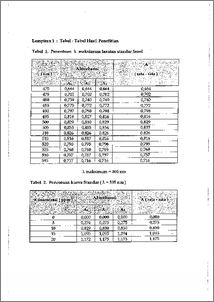
Dalam air limbah industri, banyak komponen fenol yang bersifat racun. Karena itu pada penelitian ini dicoba untuk menghilangkan komponen fenol dengan cara proses enzimatik. Enzim yang digunakan adalah peroksidase. Peroksidase yang digunakan untuk penanganan limbah fenol berasal dan jenis tumbuhan yaitu iobak ( Horseradish ). Berdasarkan hasil penelitian penanganan limbah fenol dengan peroksidase penurunan kadar fenol dalam air limbah meningkat dengan bertambahnya jumlah aktifitas enzim ( unit/mL ). Dari hasil ekperimen, enzim yang diperoleh dari ekstrak kasar ( Ek) dan hasil dialisis dan kejenuhan dengan amonium suifat 0 - 5 % ( F1 ), 5 - 20 % ( F2 ), 20 - 40 % ( F3 ), 40 - 65 % ( F4 ), dapat menurunkan kadar fenol dalam limbah yang semula 1, 068 ppm masing -masing menjadi 0,689 ppm ( 35,53 %) untuk Ek, 0,436 ppm ( 59,13 %) untuk F1, 0,641 ppm ( 39,96 % ) untuk F2, 0,736 ppm ( 31,09 % ) untuk F3 dan 0,783 ppm ( 26,66 %) untuk F4. In industrial waste water, each of this compounds is toxic. There are much fenol compounds in industrial waste water. Therefore in this research has been tried to reduce of phenol compounds by enzymatic process. The enzyme used is peroxidase which was found from lobak ( Horseradish ). From this research of the treatment of phenol waste by using peroxidase showed that the increasing concentration of enzyme would reduced of phenol. By this experiment, enzyme that was taken from crude extract ( Ek ), from dialysis and from fractination of horseradish 0 - 5 % ( Fl ), 5 - 20 % ( F2 ), 20 - 40 % ( F3 ), 40 - 65 % ( F4 ), can reduced phenol concentration in waste from 1,068 ppm to 689 ppm ( 35,53 % ) for Ek, 0,436 ppm ( 59,13 % ) for F1, 0,641 ppm ( 39,96 % ) for F2, 0,736 ppm ( 31,09 % ) for F3 and 0,783 ppm ( 26,66 `)/0 ) for F4, respectively. iv this document is Undip Institutional Repository Collection. The author(s) or copyright owner(s) agree that UNDIP-IR may, without changing the content, translate the submission to any medium or format for the purpose of preservation. The author(s) or copyright owner(s) also agree that UNDIP-IR may keep more than one copy of this submission for purposes of security, back-up and preservation. ( http://eprints.undip.ac.id) •
| Item Type: | Thesis (Undergraduate) |
|---|---|
| Subjects: | Q Science > QD Chemistry |
| Divisions: | Faculty of Science and Mathematics > Department of Chemistry |
| ID Code: | 30689 |
| Deposited By: | Mr UPT Perpus 1 |
| Deposited On: | 04 Nov 2011 13:11 |
| Last Modified: | 04 Nov 2011 13:11 |
Repository Staff Only: item control page