Hasanah , Emi (1997) Penurunan stabilitas kapsul tetrasiklin hidroklorida pada kondisi penyimpanan yang berbeda. Undergraduate thesis, FMIPA UNDIP.
| PDF Restricted to Repository staff only 2417Kb | ||
| PDF 15Kb | |
| PDF 400Kb | |
| PDF 476Kb | |
| PDF 409Kb | |
| PDF 676Kb | |
| PDF 479Kb | |
| PDF Restricted to Repository staff only 587Kb | ||
| PDF 344Kb | |
| PDF 373Kb | |
| PDF 1029Kb |
Abstract
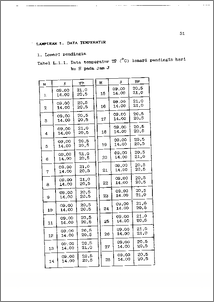
Telah dilakukan penentuan kandungan bahan aktif tetrasiklin hidroklorida di dalam kapsul tetrasiklin hidroklorida dengan menggunakan spektrofotometer ultraviolet-visible pada panjang gelombang 381 nm. Kandungan bahan aktif dalam sediaan obat adalah salah satu sifat khas kualitas. Dengan kondisi penyimpanan yang berbeda dan menentukan kandungan bahan aktifnya akan didapatkan gambaran stabilitas kapsul tetrasiklin hidroklorida. Penelitian dilaksanakan dengan Rancangan Acak Lengkap Pola Faktorial 9 X 7 dengan ulangan 3 kali. Faktor A adalah kondisi penyimpanan terdiri atas : S = Standar (kapsul dengan kemasannya dalam botol coklat tertutup di lemari pendingin); DRC = kapsul dengan kemasannya dalam botol coklat tertutup di dalam ruangan; DRB = kapsul dengan kemasannya dalam botol bening di dalam ruangan; TRC = kapsul tanpa kemasan dalam botol coklat tertutup di dalam ruangan; TRB = kapsul tanpa kemasan dalam botol bening di dalam ruangan; DC = kapsul dengan kemasannya dalam botol coklat tertutup di ruangan terbuka; DB = kapsul dengan kemasannya dalam botol bening di ruangan terbuka; TC = kapsul tanpa kemasan dalam botol coklat tertutup di ruangan terbuka dan TB = kapsul tanpa kemasan dalam botol bening di ruangan terbuka. Faktor B adalah lama penyimpanan terdiri atas : 0 = 0 hari; 3 = 3 hari; 7 = 7 hari; 10 = 10 hari; 14 = 14 hari; 2/ = 21 hari dan 28 = 28 hari. Data kandungan bahan aktif sebagai amatan yang diperoleh, dianalisis dengan menggunakan Sidik Ragam dan untuk menguji perbedaan antar perlakuan digunakan Uji Wilayah Duncan. Hasil penelitian menunjukkan bahwa kondisi dan lama penyimpanan beserta interaksinya berpengaruh sangat nyata terhadap kandungan bahan aktif tetrasiklin hidroklorida. Peningkatan lama penyimpanan menurunkan kandungan bahan aktif. Penurunan yang te•besar diperoleh pada perlakuan DB yaitu penyimpanan kapsul tetrasiklin hidroklorida dengan kemasannya dalam botol bening di ruangan terbuka. Kandungan bahan aktif tetrasiklin hidroklorida dalam 10 ppm sampel kaspsul tetrasiklin hidroklorida dari perlakuan DB selama penyimpanan 0; 3; 7; 10; 14; 21 dan 28 hari adalah masing-masing : 9,73; 8,77; 8,73; 8,67; 8,73; 7,77 dan 7,27 ppm. The content of active substrate tetracyclin hydrochloride from tetracyclin hydrochloride capsule has been determined by Spectrophotometer UV-Vis in wavelength of 381 nm. The content of active substrate of drug is a kind of quality specific characteristic. By different storage followed by determination active substrate content will get a description of stability of tetracyclin hydrochloride capsule. Reseach was done by Complete Random Design, Factorial 9 X 7 with 3 repetitions. The A factor is storage condition, consists of : S = Standard (packed capsul in capped brown bottle in refrigerator); DRC = packed capsul in capped brown bottle in room condition; DRB = packed capsul in transparent bottle in room condition; TRC = unpacked capsul in capped brown bottle in room condition; TRB = unpacked capsul in transparent bottle in room condition; DC = packed capsul in capped brown bottle in open room condition; DB = packed capsul in transparent bottle in open room condition; TC = unpacked capsul in capped brown bottle in open room condition and TB = unpacked capsul in transparent bottle in open room condition. The B factor is time of storage, consists of 0= 0 day; 3 = 3 days; 7 = 7 days; 10 = 10 days; 14 = 14 days; 21 = 21 days and 28 = 28 days. The Data of active substrate content resulted was analyzed by Analysis of Variance and tested the treatment differencess by Duncan Range. The result of the treatment differencess reseach showed that the condition and time of storage and its interaction as well affected the content of active substrate significantly. The increase time of storage reduced the content of active substrate. The most rapid decrease was got on treatment of DB namely storage of packed tetracyclin hydrochloride capsule in transparent bottle in the open room. The Content of active substrate of tetracyclin hydrochloride in sample of 10 ppm of teracyclin hydrochloride capsule of DB treatment for 0 day; 3 days; 7 days; 10 days; 14 days; 21 days and 28 days storage were : 9,73; 8,77; 8,73; 8,67; 8,73; 7,77 and 7,27 ppm.
| Item Type: | Thesis (Undergraduate) |
|---|---|
| Subjects: | Q Science > QD Chemistry |
| Divisions: | Faculty of Science and Mathematics > Department of Chemistry |
| ID Code: | 30637 |
| Deposited By: | Mr UPT Perpus 1 |
| Deposited On: | 04 Nov 2011 08:03 |
| Last Modified: | 04 Nov 2011 08:03 |
Repository Staff Only: item control page