Astuti , Ardini (1997) Adsorsi zat warna metilena biru pada karbon aktif dalam larutan sukrosa. Undergraduate thesis, FMIPA UNDIP.
| PDF Restricted to Repository staff only 1949Kb | ||
| PDF 20Kb | |
| PDF 360Kb | |
| PDF 491Kb | |
| PDF 371Kb | |
| PDF 707Kb | |
| PDF 446Kb | |
| PDF Restricted to Repository staff only 626Kb | ||
| PDF 345Kb | |
| PDF 356Kb | |
| PDF 605Kb |
Abstract
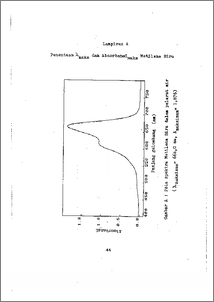
Adsorpsi dengan karbon aktif banyak digunakan sebagai cara untuk memurnikan zat terhadap warna, rasa, dan bau. Kemampuan adsorpsi karbon aktif ditentukan oleh luas permukaan, ukuran pori dan komposisi kimia (gugus fungsi), yang dapat dipengaruhi oleh pH dan suhu. Penelitian ini sebagai pemodelan untuk mempelajari pengaruh pH dan suhu pada daya serap karbon aktif terhadap zat warna metilena biru dalam larutan sukrosa 20 % b/v, dengan metode spektrofotometri ultraviolet dan sinar tampak. Dalam penelitian ini diperoleh pH yang efektif untuk adsorpsi adalah pH = 8 - 9, dengan konsentrasi meti¬lena biru terserap 1,397 - 1,402 ppm dan % penghilangan warna 93,13 - 93,47 % . Suhu adsorpsi efektif adalah 60 -¬80 C, dengan konsentrasi metilena biru terserap 1,491 -1,495 ppm dan % penghilangan warna 99,40 - 99,67 %. Dari hasil penelitian dapat disimpulkan bahwa pH dan suhu dapat diatur guna meningkatkan daya serap karbon aktif terhadap zat warna metilena biru dalam larutan sukrosa, ka•ena pH dan suhu dapat mempengaruhi kemampuan adsorpsi karbon aktif, kestabilan zat warna dan produk. Adsorption with activated carbon was widely appli¬cated as method for materials purification from colour,. taste, and odor. Power adsorption of activated carbon was affected by surface area, pore size and chemical composition ( fun - ctional groups at activated carbon surfaces) which could be influenced by pH and temperature. This experiment as model for study of the influ ence of pH and temperature on decolorizing power of activa¬ted carbon from methylene blue dye solution in sucrose so - lution 20 % w/v with spectrophotometry -UV/Visible'method. The experiment results effective pH for adsorption was pH = 8 - 9, with concentration of adsorption methylene blue 1,397 - 1,402 ppm and % decolorization is 93,13 - 93647 %. The effective temperature of adsorption was 60 -85 C with concentration adsorption of methylene blue was 1,491 - 1,495 ppm and % decolorization is 99,40-99,67 %. From the experiment result was concluded that pH and temperature could be regulated for the increase of ad¬sorption power of activated carbon of dye in sucrose solu¬tion, because pH and temperature can affect activated car¬bon power adsorption, stability of dye and products.
| Item Type: | Thesis (Undergraduate) |
|---|---|
| Subjects: | Q Science > QD Chemistry |
| Divisions: | Faculty of Science and Mathematics > Department of Chemistry |
| ID Code: | 30635 |
| Deposited By: | Mr UPT Perpus 1 |
| Deposited On: | 04 Nov 2011 07:54 |
| Last Modified: | 04 Nov 2011 07:54 |
Repository Staff Only: item control page