Agustini , Dewi Meliati (1997) Penggunaan CaOH)2 dalam daur ulang Trietil amina pada pembuatan Ampisilin Trihidrat dan Amoksisilin Trihidrat. Undergraduate thesis, FMIPA UNDIP.
| PDF 426Kb | |
| PDF 363Kb | |
| PDF Restricted to Repository staff only 1168Kb | ||
| PDF 601Kb | |
| PDF 970Kb | |
| PDF 406Kb | |
| PDF 586Kb | |
| PDF 417Kb | |
| PDF 17Kb | |
| PDF Restricted to Repository staff only 4Mb | ||
| PDF 1884Kb |
Abstract
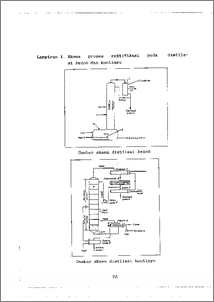
Cairan induk dari pembuatan Ampisilin Trihidrat (eks. AMP) dan Amoksisilin Trihidrat (eks. AMX) mengandung Trie¬tilamina (TEA) dalam bentuk garam kloridanya (TEA-HC1). Daur ulang TEA telah dilakukan dengan teknik distilasi pada pH > 12 dengan penambahan NaOH 48% dan menghasilkan garam anorganik terlarut (ROI) yang tinggi (± 800 - 1000 kg/hart) dalam air limbah olahan. Sehingga dalam penelitian ini dicoba untuk menggantikan NaOH 48% dengan Ca(OH)2 teknis dan campuran Ca(OH)2 teknis-NaOH 48%. Cairan induk dari sintesis (ML) dan dari bagian daur ulang (FML) balk eks. AMP dan eks. AMX masa produksi IV sampai VI dicoba direge¬nerasi pada pH 12,5 untuk Skala laboratorium dengan tiga perlakuan yaitu dengan NaOH 48% (perlakuan 1), dengan Ca(OH)2 teknis (perlakuan 2) dan dengan campuran Ca(OH)2 teknis--NaOH 48% (perlakuan 3) yang kemudian didistilaei dan masing-masing TEA-1 yang diperoleh dibebaskan airnya dengan NaOH padat menjadi TEA-2. Semua perlakuan di atas bertujuan untuk mengetahui perbandingan kualitas TEA-1 serta kuanti¬tas TEA-1 dan TEA-2 yang diperoleh dari parameter kadar pengotor dan volume; perbedaan kuantitas penggunaan basa¬nya, ROI, dan kuantitas endapan; serta karakteristik ma¬sing-masing residu terhadap kualitas air limbah dari para¬meter kimia yaitu Kebutuhan Oksigen Kimia (KOK), Total Kjeldahl Nitrogen (TKN), kadar ROI dan alkalinitas serta parameter biokimia yaitu uji laju pemakaian oksigen (OUR). Hasil penelitian menunjukkan bahwa perlakuan 2 memberikan kualitas TEA-1 yang sama dengan perlakuan 1 dan 3 serta memberikan kuantitas TEA-1 dan TEA-2 yang lebih kecil; lebih sedikit menggunakan basa sehingga lebih ekonomis dan lebih kecil kadar R0I-nya; dan hasil analisis parameter kimia menunjukkan karakteristik filtrat residu yang lebih balk terhadap kualitas air limbah dibandingkan residu/filtrat residu perlakuan 1 dan 3 serta filtrat residu perlakuan 2 dapat didegradasi secara biologic. Hasil analisis rancangan 3 faktor dan analisis rancangan 2 faktor terhadap data volume residu (Vr), konsentrasi KOK serta TKN, kadar ROI dan alkalinitas menunjukkan bahwa ML berbeda dengan FML serta rata-rata dari parameter kimia perlakuan 2 berbeda nyata dengan perlakuan 1 dan 3 sedangkan antara perlakuan 1 dan 3 tidak berbeda nyata. Sehingga dapat disimpulkan bahwa Ca(OH)2 dapat menggantikan NaOH 48% untuk regenerasi TEA-HCI tetapi tidak untuk skala pabrik karena endapan yang dihasilkan dan harus dipertimbangkan lebih lanjut. Mother liquor from trihydrate of Ampicillin (ex. AMP) and Amoxicillin (ex. AMX) production contains triethyla¬mine (TEA) in the form of chloride salt (TEA-HC1). Recy¬cling process of TEA has been done by distillation tech¬nique at pH > 12 with an addition of NaOH 48% and produces a high soluble inorganic salts (ROI) (± 800 - 1000 kg/day) in waste water. So in this research technical grade Ca(OH)2 and mixture of technical grade Ca(OH)2-NaOH 48% was tried to replace NaOH 48%. Mother liquor from synthesis (ML) and tank farm division (FML) on their IV until VI campaign production were tried in the laboratory scale with three treatment that was regenerated at pH 12.5 by addition of NaOH 48% (treatment 1), technical grade Ca(OH)2 (treatment 2) and mixture of technical grade Ca(OH)2-NaOH 48% (treat¬ment 3) and then all of them were distiliated. The content of water of each TEA-1 results were released by addition of NaOH flakes as TEA-2. All these treaments had purpose to find out the comparison of TEA-1 quality and quantity of TEA-1 and TEA-2 which were resulted with impurities content and volume parameter; the difference of quantity of base consuming, ROI content and precipitate quantity; and the characteristic of residue from each distillation process toward the waste water quality with chemical parameters such Chemical Oxygen Demand (COD), Total Kjeldahl Nitrogen (TKN), ROI content and alkalinity, and with Oxygen Up take Rate (OUR) test as biochemical parameter. The result showed that treatment 2 gave an equal result of TEA-1 quality with TEA-1 of treatment 1 and 3, and gave smaller quantity of TEA-1 and TEA-2 too; and also consumed less slightly base and more economize and ROI content was smaller; and the re¬sults of analysis chemical parameters showed characteristic of residue filtrate toward the waste water quality was better than residue/residue filtrate of treatment 1 and 3, and it also biologically degradated. The results of analy¬sis of 3 factorial design and of 2 factorial design toward datas of the residue volume (Vr), COD and TKN concentra¬tion, ROI content and alkalinity showed that ML was diffe¬rent with FML, and average of analysis results of chemical parameters for residue filtrate of treatment 2 denoted a statistically significant difference with results of treat¬ment 1 and 3, where as the results between treatment 1 and 3 did not. So, technical grade Ca(OH)2 could replace NaOH 48% to regenerate of TEA-HCI but it would not do in the real plant scale because a precipitate was produced in its residue and must be further considered. ,..Dnal Repository Collection. The author(s) or copyri8. !IL cu 6.&.i, submissicin to any medium or format for the purpose of preservation. The author(s) or copyright owner(s) also agree that UNDIP-IR may keep more than one copy of this submissijn for purposes ,.urity, back-up and preservation..1 htto//eprintS..141:14Lar-id-)
| Item Type: | Thesis (Undergraduate) |
|---|---|
| Subjects: | Q Science > QD Chemistry |
| Divisions: | Faculty of Science and Mathematics > Department of Chemistry |
| ID Code: | 30631 |
| Deposited By: | Mr UPT Perpus 1 |
| Deposited On: | 04 Nov 2011 07:38 |
| Last Modified: | 04 Nov 2011 07:38 |
Repository Staff Only: item control page