Purwaningrum , Widia (1996) Penurunan kadar flavonoid dari biji kakao setelah fermentasi (theobroma cacao L). Undergraduate thesis, FMIPA UNDIP.
| PDF Restricted to Repository staff only 1949Kb | ||
| PDF 17Kb | |
| PDF 361Kb | |
| PDF 509Kb | |
| PDF 384Kb | |
| PDF 767Kb | |
| PDF 479Kb | |
| PDF 768Kb | |
| PDF Restricted to Repository staff only 768Kb | ||
| PDF 348Kb | |
| PDF 361Kb | |
| PDF 323Kb |
Abstract
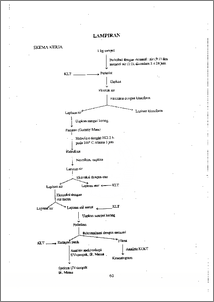
Dalam biji kakao terdapat senyawa polifenol kira-kira 12-18% . Selama fermentasi dan pengeringan, terjadi perubahan kimia polifenol, yang mempengaruhi warna dan rasa kakao. Isolasi senyawa flavonoid, yang merupakan bagian dari senyawa polifenol, dari keping biji kakao lindak fermentasi 5 hari. Ekstraksi dilakukan menggunakan campuran metanol dan air (9:1) dan campuran metanol dan air (1:1). Selanjutnya dilakukan hidrolisis menggunakan HC1 2N. Aglikon yang diperoleh diekstraksi dengan eter dan kemudian etil asetat. Pemurnian hasil dilakukan dengan rekristalisasi menggunakan pelarut metanol. Analisa hasil dilakukan dengan metoda kromatografi lapis tipis dua dimensi menggunakan TBA (3:1:1) dan TBA (1:1:3) sebagai eluen. Spektroskopi ultraviolet-tampak, inframerah dan massa dilakukan untuk menentukan struktur hasil isolasi. Dari data yang diperoleh diduga bahwa senyawa hasil isolasi yang berupa padatan adalah theobromin. Sedangkan terhadap filtratnya dilakukan analisis Kromatografi Cair Kinerja Tinggi dengan metoda waktu retensi analit terhadap waktu retensi acuan pembandingan. Hasil analisis di atas menunjukkan bahwa dalam keping biji kakao lindak fermentasi 5 hari terjadi pengurangan kadar flavonoid dalam biji kakao yang masih menyumbang rasa menggigit dan pahit. In cocoa beans, polyphenols approximately consist of 12-18 % from whole weight. During fermentation and drying, chemical changes in polyphenol occur which affect the flavor and the colour of chocolate. Isolation of flavonoid compound, which is a part of polyphenol compound, from Forastero nib fermented 5 days. Extraction was done using methanol.: water (9:1) and methanol : water (1:1) as solvent. Then the hydrolisis was done using HC1 2N. Aglicon that we had was extracted with ether and followed by ethyl acetate. To get the pure product was done by recrystalisation using methanol and analyzed by two dimension chromathography method using TBA (3:1:1) and TBA (1:1:1) as eluent. To show the structure of the isolation product, ultraviolet-visible spectroscopy, infrared spectroscopy and mass spectrometry were used. And suggested from the data, the compound is theobromin. Further, high pressure liquid chromathography was used to analyze the filtrate by a method of comparing retention time between reference and analyze retention time. This document is Undip Institutional Repository Collection. The author(s) or copyright owner(s) agree that UNDIP-IR may, without changing the content, translate the subrriission to any medium or format for the purpose of preservation. The author(s) or copyright owner(s) also agree that UNDIP-IR may keep more than one copy of this • subnlission for purposes of security, back-up and preservation. ( http://eprints.undip.ac.id)
| Item Type: | Thesis (Undergraduate) |
|---|---|
| Subjects: | Q Science > QD Chemistry |
| Divisions: | Faculty of Science and Mathematics > Department of Chemistry |
| ID Code: | 30620 |
| Deposited By: | Mr UPT Perpus 1 |
| Deposited On: | 03 Nov 2011 12:08 |
| Last Modified: | 03 Nov 2011 12:08 |
Repository Staff Only: item control page