Rusmanto , Rusmanto (1994) Pengaruh kadar Si di dalam air tanah dengan magnesium. Undergraduate thesis, FMIPA UNDIP.
| PDF Restricted to Repository staff only 1662Kb | ||
| PDF 14Kb | |
| PDF 342Kb | |
| PDF 479Kb | |
| PDF 357Kb | |
| PDF 606Kb | |
| PDF 449Kb | |
| PDF Restricted to Repository staff only 478Kb | ||
| PDF 340Kb | |
| PDF 336Kb | |
| PDF 636Kb |
Abstract
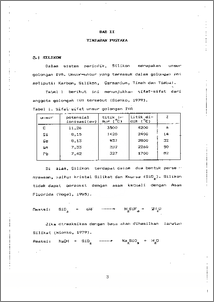
Telah dilakukan penelitian mengenai pengurangan kadar Si dalam air tanah dengan menggunakan Magnesium. Pengamatan terhadap perubahan konsentrasi- ini diikuti secara spektrofotometrik memakai spektronik-20, yang diatur pada panjang gelombang 720 nm. Konsentrasi awal Si dalam air tanah 99,73744 ppm. Penelitian dilakukan dalam suhu 27°C dengan variabel pH (9,5-11,5), variabel konsentrasi Magnesium Oksida (100-500 ppm) dan variabel lama waktu pengadukan (5-60) menit. Hasil penelitian menunjukkan bahwa pengurangan kadar Si optimum diperoleh pada pH 11,5, konsentrasi Mg0 200 ppm dan pengadukan selama 30 menit. Pada kondisi ini hampir 97,07. kandungan Si dalam air dapat terambil. Jadi pH, konsentrasi MgO dan pengadukan mempengaruhi pengurangan Si dalam air tanah. The experiment decreasing of Silicon concentration in ground water by Magnesium was done. The change in the concentration of Silicon measured by spectronic-20, netted at wave length 720 nm. The initial concentration of Silicon is 99.73744 ppm. The experiment carried out at 27°C with pH variable (9.5-11.5), Magnesium Oxide (100- 500) ppm and time of stirring variable (5-60) minutes. The result of experiment indicated that the removal of Silicon by Magnesium is much more efficient and flexible in pH 11.5, Magnesium Oxide concentration 200 ppm and stirring as long as 30 minutes. In this condition nearly 97.07. Silicon concentration removed. So pH, Magnesium Oxide concentration and stirring determine of the decrease Silicon concentration in ground water.
| Item Type: | Thesis (Undergraduate) |
|---|---|
| Subjects: | Q Science > QD Chemistry |
| Divisions: | Faculty of Science and Mathematics > Department of Chemistry |
| ID Code: | 30573 |
| Deposited By: | Mr UPT Perpus 1 |
| Deposited On: | 02 Nov 2011 15:37 |
| Last Modified: | 02 Nov 2011 15:37 |
Repository Staff Only: item control page