Yulianto , Arman (1994) Penurunan konsentrasi Ci pada air dengan fitrasi pasir besi. Undergraduate thesis, FMIPA UNDIP.
| PDF Restricted to Repository staff only 1603Kb | ||
| PDF 15Kb | |
| PDF 345Kb | |
| PDF 406Kb | |
| PDF 378Kb | |
| PDF 589Kb | |
| PDF 419Kb | |
| PDF Restricted to Repository staff only 420Kb | ||
| PDF Restricted to Repository staff only 488Kb | ||
| PDF 326Kb | |
| PDF 330Kb | |
| PDF 576Kb |
Abstract
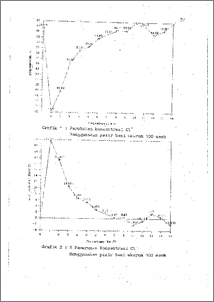
Telah diteliti kemampuan pasir besi untuk mengurangi konsentrasi Cl dengan variabel berubah adalah jenis pasir (pasir kwarsa Muntilan dan pasir besi), ukuran partikel pasir besi (60 mesh, 80 mesh , 100 mesh) dan konsentrasi NaCI yang disaring (100 mg/1, 150 mg/1, 200 mg/1). Hasil yang didapat adalah semakin kecil partikel pasir besi pada volume yang sama, maka kemampuan penyerapan Cl -nya makin bagus, serta penyerapan yang paling banyak adalah penyerapan pertama dengan menggunakan pasir besi ukuran 100 mesh, yaitu 26.07 % . Dibanding dengan pasir kwarsa Muntilan maka pasir besi lebih banyak menyerap Cl pada ukuran partikel yang sama, yaitu pasir Muntilan sebanyak 7.17 % s/d 10.11 % dan pasir besi sebanyak 12.83 % s/d 17.67 % . Dari pengukuran pH didapat data bahwa setelah penyaringan terjadi kenaikan pH. The ability of iron sands to decrease Cl- concentration had been examined, with independent variables : variety of sands (Muntilan's sands, iron sands), particle sizes of iron sands (60 mesh, 80 mesh, 100 mesh) and concentration of NaC1 which to be filtrated (100 mg/1, 150 mg/1, 200 mg/I). Found from the result that the smaller particle size of iron sands (in same volume) would be better adsorpstivity of iron sands. The first filtration with 100 mesh iron sands was the optimum adsorption,that was 26,07 % . The iron sands was better than Muntilan's sands in adsorpstivity of Cl concentration of the same particle size (60 mesh), absorptivity of Muntilan's and iron sands 7.17 % to 10.11 %arid 12.63 % to 17.67 % respectively. The result of pH measuring showed that pH increase after filtratio
| Item Type: | Thesis (Undergraduate) |
|---|---|
| Subjects: | Q Science > QD Chemistry |
| Divisions: | Faculty of Science and Mathematics > Department of Chemistry |
| ID Code: | 30572 |
| Deposited By: | Mr UPT Perpus 1 |
| Deposited On: | 02 Nov 2011 15:33 |
| Last Modified: | 02 Nov 2011 15:33 |
Repository Staff Only: item control page